Abstract
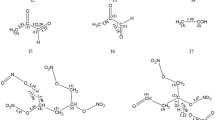
Electronic structure methods based on quantum mechanics were employed to characterize elementary steps for the gas-phase thermal decomposition of triazene-bridged nitro-1,2,4-triazole (TBBT). Homolytic C–NO2 bond scission and ·NO2 elimination were the most energetically favorable unimolecular paths for the initial decomposition. From there, sequences of unimolecular reactions for daughters of the initiation steps through low-energy β-scission reactions and ring-opening reaction were postulated and characterized. Hydron shift, C–N bond breakage, nitrogen and NO2 elimination, and small molecules like CN–N=NH obtained were all characterized. Creating a comprehensive network that can be used to develop a detailed limited rate chemical dynamic mechanism for simulating decomposition of TBBT, the results provide the foundation for TBBT’s combustion modeling, and response to its aging, and storage.













Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Dippold AA, Klapötke TM (2013) A study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides. J Am Chem Soc 135:9931–9938. https://doi.org/10.1021/ja404164j
Thottempudi V, Shreeve JM (2011) Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole. J Am Chem Soc 133:19982–19992. https://doi.org/10.1021/ja208990z
Zhang J, Dharavath S, Mitchell LA, Parrish DA, Shreeve JNM (2013) Energetic salts based on 3,5-bis(dinitromethyl)-1,2,4-triazole monoanion and dianion: controllable preparation, characterization, and high performance. J Am Chem Soc 138:7500–7503. https://doi.org/10.1021/jacs.6b03819
Klapötke TM, Minar NK, Stierstorfer J (2009) Investigations of bis (methyltetrazolyl) triazenes as nitrogen-rich ingredients in solid rocket propellants–synthesis, characterization and properties. Polyhedron 28(1):13–26. https://doi.org/10.1016/j.poly.2008.09.015
Feng S, Li F, Zhao X, Qian YD, Fei T, Yin P, Pang SP (2021) Comparative study on 1,2,3-triazole based azo-and triazene-bridged high-nitrogen energetic materials. Energ Mater Front 2:125–130. https://doi.org/10.1016/j.enmf.2021.03.006
Jiang X, Yang Y, Du H, Yang B, Tang P, Wu B, Ma C (2023) Triazene bridged energetic materials based on nitrotriazole: synthesis, characterization and laser ignited combustion performance. Dalton Trans 52:5226–5233. https://doi.org/10.1039/D2DT04007G
Jiang X, Li L, Yang Y, Du H, Wang Y, Zhu J, Ma C (2023) Boosting laser-ignited combustion performance of energetic materials with low sensitivity: integration of triazene-bridged triazole with oxygen-rich moieties via noncovalent self-assembly. Cryst Growth Des 23:333–341. https://doi.org/10.1021/acs.cgd.2c01039
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Rev. C.01. Gaussian Inc., Wallingford
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Lu T, Chen Q (2021) Interaction region indicator: a simple real space function clearly revealing both chemical bonds and weak interactions. Chem Methods 1:231–239. https://doi.org/10.1002/cmtd.202100007
Veals JD, Chen CC (2021) Thermal decomposition of gas-phase bis(1,2,4-oxadiazole)bis(methylene) dinitrate (BODN): ACCSD(T)-F12/DFT-based study of reaction pathways. J Phys Chem A 125:9077–9091. https://doi.org/10.1021/acs.jpca.1c06065
Acknowledgements
This research was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20220352). We are so grateful to the High-Performance Computing Center of Nanjing Tech University for doing the numerical calculations in this paper on its x-Flex enterprise blade cluster system.
Author information
Authors and Affiliations
Contributions
Congming Ma helped in conceptualization, original draft, and funding acquisition. Kehan Hu contributed to writing—original draft. Peng Ma helped in methodology and supervision. Wenxin Xia contributed to writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, C., Hu, K., Ma, P. et al. Mechanistic investigation on the gas-phase thermal decomposition of triazene-bridged nitro-1,2,4-triazole. Theor Chem Acc 143, 45 (2024). https://doi.org/10.1007/s00214-024-03120-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03120-1