Abstract
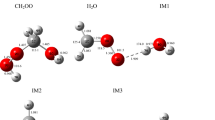
Gas-phase reactions involving simplest Criegee intermediate (CH2OO) have been the current hot topic due to its vital role in atmospheric chemistry. In this study, high-level ab initio calculations are used to investigate the energetics and kinetics for the reaction of CH2OO + ROH → ROCHO + H2O (R=CH3, CH3CH2 and (CH3)2CH). Energies of the stationary points are computed at the CCSD(T)/M06-2X/6-311++G(3d,3pd)//M06-2X/6-311++G(3d,3pd) level of theory. Reaction is going through a 1,2-addition and water elimination step leading to the formation of alkoxymethyl hydroperoxides and alkyl formates, respectively. The barrier heights for the 1,2-addition step with methanol, ethanol, and isopropanol were found to be − 3.1, − 3.7, and − 4.8 kcal mol−1, and water elimination steps were found to be 2.2, 1.5, and 1.6 kcal mol−1, respectively, relative to the energies of the starting reactants. The rate constants for addition and elimination channels were calculated using canonical variational transition state theory in conjugation with small-curvature tunneling and the interpolated single point energy method between the temperature range of 200 and 500 K. In addition, the thermochemistry analysis indicates that addition and elimination channels are thermodynamically feasible and the formation of alkyl formates is entropically more favored when compared to the formation of alkoxymethyl hydroperoxide along the reaction path in the potential energy surface. The pressure-dependent microcanonical rate constants for both addition and elimination channels were also estimated using the Rice–Ramsperger–Kassel–Marcus theory and discussed in this study.









Similar content being viewed by others
Data availability
All data obtained through this computation are tabulated in Supporting Information.
References
Goldan PD, Kuster WC, Fehsenfeld FC, Montzka SA (1993) The observation of a C5 alcohol emission in a North American pine forest. Geophys Res Lett 20:1039–1042
Stavrakou T, Guenther A, Razavi A et al (2011) First space-based derivation of the global atmospheric methanol emission fluxes. Atmos Chem Phys 11:4873–4898. https://doi.org/10.5194/acp-11-4873-2011
Grosjean E, Grosjean D, Gunawardena R, Rasmussen RA (1998) Ambient concentrations of ethanol and methyl tert-butyl ether in Porto Alegre, Brazil, March 1996–April 1997. Environ Sci Technol 32:736–742. https://doi.org/10.1021/es970788u
Grosjean E, Rasmussen RA, Grosjean D (1998) Ambient levels of gas phase pollutants in Porto Alegre, Brazil. Atmos Environ 32:3371–3379. https://doi.org/10.1016/S1352-2310(98)00007-7
Jacob DJ, Field BD, Li Q et al (2005) Global budget of methanol: constraints from atmospheric observations. J Geophys Res D Atmos 110:1–17. https://doi.org/10.1029/2004JD005172
Heikes BG, Chang W, Pilson MEQ et al (2002) Atmospheric methanol budget and ocean implication. Glob Biogeochem Cycles. https://doi.org/10.1029/2002gb001895
Sivapuram MS, Nagarathna R, Anand A et al (2020) Prevalence of alcohol and tobacco use in india and implications for COVID-19-Niyantrita Madhumeha Bharata Study projections. J Med Life 13:499–509. https://doi.org/10.25122/jml-2020-0079
Kirstine WV, Galbally IE (2012) ethanol in the environment: a critical review of its roles as a natural product, a biofuel, and a potential environmental pollutant. Crit Rev Environ Sci Technol 42:1735–1779
Colón M, Pleil JD, Hartlage TA et al (2001) Survey of volatile organic compounds associated with automotive emissions in the urban airshed of São Paulo, Brazil. Atmos Environ 35:4017–4031. https://doi.org/10.1016/S1352-2310(01)00178-9
Nguyen HTH, Takenaka N, Bandow H et al (2001) Atmospheric alcohols and aldehydes concentrations measured in Osaka, Japan and in Sao Paulo, Brazil. Atmos Environ. https://doi.org/10.1016/S1352-2310(01)00136-4
König G, Brunda M, Puxbaum H et al (1995) Relative contribution of oxygenated hydrocarbons to the total biogenic VOC emissions of selected mid-European agricultural and natural plant species. Atmos Environ 29:861–874. https://doi.org/10.1016/1352-2310(95)00026-U
Docherty KS, Ziemann PJ (2003) effects of stabilized criegee intermediate and OH radical scavengers on aerosol formation from reactions of β-pinene with O3. Aerosol Sci Technol 37:877–891. https://doi.org/10.1080/02786820300930
Wolff S, Boddenberg A, Thamm J et al (1997) Gas-phase ozonolysis of ethene in the presence of carbonyl-oxide scavengers. Atmos Environ 31:2965–2969. https://doi.org/10.1016/S1352-2310(97)00114-3
Criegee R (1975) Mechanism of ozonolysis. Angew Chem Int Ed Engl 14:745–752. https://doi.org/10.1002/anie.197507451
Johnson D, Marston G (2008) The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem Soc Rev 37:699–716. https://doi.org/10.1039/b704260b
Taatjes CA, Shallcross DE, Percival CJ (2014) Research frontiers in the chemistry of Criegee intermediates and tropospheric ozonolysis. Phys Chem Chem Phys 16:1704–1718
Calvert JG, Atkinson R, Kerr JA et al (2002) The mechanisms of atmospheric oxidation of the aromatic hydrocarbons
Horie O, Moortgat GK (1998) Gas-phase ozonolysis of alkenes. Recent advances in mechanistic investigations. Acc Chem Res 31:387–396. https://doi.org/10.1021/ar9702740
Ke L, McGillen MR, Cooke MC et al (2012) Acid-yield measurements of the gas-phase ozonolysis of ethene as a function of humidity using Chemical Ionisation Mass Spectrometry (CIMS). Atmos Chem Phys 12:469–479. https://doi.org/10.5194/acp-12-469-2012
Welz O, Savee JD, Osborn DL et al (2012) Direct kinetic measurements of criegee intermediate (CH2OO) formed by reaction of CH2I with O2. Science 335:204–207. https://doi.org/10.1126/science.1213229
Liu F, Beames JM, Green AM, Lester MI (2014) UV spectroscopic characterization of dimethyl- and ethyl-substituted carbonyl oxides. J Phys Chem A 118:2298–2306. https://doi.org/10.1021/jp412726z
Beames JM, Liu F, Lu L, Lester MI (2013) UV spectroscopic characterization of an alkyl substituted Criegee intermediate CH3CHOO. J Chem Phys. https://doi.org/10.1063/1.4810865
Lin HY, Huang YH, Wang X et al (2015) Infrared identification of the Criegee intermediates syn- and anti-CH3CHOO, and their distinct conformation-dependent reactivity. Nat Commun. https://doi.org/10.1038/ncomms8012
Wang YY, Chung CY, Lee YP (2016) Infrared spectral identification of the Criegee intermediate (CH3)2COO. J Chem Phys. https://doi.org/10.1063/1.4964658
Smith MC, Chang CH, Chao W et al (2015) Strong negative temperature dependence of the simplest criegee intermediate CH2OO reaction with water dimer. J Phys Chem Lett 6:2708–2713. https://doi.org/10.1021/acs.jpclett.5b01109
Huang HL, Chao W, Lin JJM (2015) Kinetics of a Criegee intermediate that would survive high humidity and may oxidize atmospheric SO2. Proc Natl Acad Sci USA 112:10857–10862. https://doi.org/10.1073/pnas.1513149112
Chhantyal-Pun R, Shannon RJ, Tew DP et al (2019) experimental and computational studies of Criegee intermediate reactions with NH3 and CH3NH2. Phys Chem Chem Phys 21:14042–14052. https://doi.org/10.1039/c8cp06810k
Ryzhkov AB, Ariya PA (2004) A theoretical study of the reactions of parent and substituted Criegee intermediates with water and the water dimer. Phys Chem Chem Phys 6:5042–5050. https://doi.org/10.1039/b408414d
Anglada JM, González J, Torrent-Sucarrat M (2011) Effects of the substituents on the reactivity of carbonyl oxides. A theoretical study on the reaction of substituted carbonyl oxides with water. Phys Chem Chem Phys 13:13034–13045. https://doi.org/10.1039/c1cp20872a
Taatjes CA, Welz O, Eskola AJ et al (2013) Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science 340:177–180. https://doi.org/10.1126/science.1234689
Misiewicz JP, Elliott SN, Moore KB, Schaefer HF (2018) Re-examining ammonia addition to the Criegee intermediate: converging to chemical accuracy. Phys Chem Chem Phys 20:7479–7491. https://doi.org/10.1039/c7cp08582f
Jørgensen S, Gross A (2009) Theoretical investigation of the reaction between carbonyl oxides and ammonia. J Phys Chem A 113:10284–10290. https://doi.org/10.1021/jp905343u
Elsamra RMI, Jalan A, Buras ZJ et al (2016) Temperature- and pressure-dependent kinetics of CH2OO + CH3COCH3 and CH2OO + CH3CHO: Direct measurements and theoretical analysis. Int J Chem Kinet 48:474–488. https://doi.org/10.1002/kin.21007
Wang YY, Dash MR, Chung CY, Lee YP (2018) Detection of transient infrared absorption of SO3 and 1,3,2-dioxathietane-2,2-dioxide [cyc -(CH2)O(SO2)O] in the reaction CH2OO+SO2. J Chem Phys. https://doi.org/10.1063/1.5019205
Tadayon SV, Foreman ES, Murray C (2018) Kinetics of the reactions between the Criegee intermediate CH2OO and alcohols. J Phys Chem A 122:258–268. https://doi.org/10.1021/acs.jpca.7b09773
McGillen MR, Curchod BFE, Chhantyal-Pun R et al (2017) Criegee intermediate-alcohol reactions, a potential source of functionalized hydroperoxides in the atmosphere. ACS Earth Space Chem 1:664–672. https://doi.org/10.1021/acsearthspacechem.7b00108
Lin LC, Chang HT, Chang CH et al (2016) Competition between H2O and (H2O)2 reactions with CH2OO/CH3CHOO. Phys Chem Chem Phys 18:4557–4568. https://doi.org/10.1039/c5cp06446e
Watson NAI, Black JA, Stonelake TM et al (2019) An extended computational study of Criegee intermediate-alcohol reactions. J Phys Chem A 123:218–229. https://doi.org/10.1021/acs.jpca.8b09349
Aroeira GJR, Abbott AS, Elliott SN et al (2019) The addition of methanol to Criegee intermediates. Phys Chem Chem Phys 21:17760–17771. https://doi.org/10.1039/c9cp03480c
Chao W, Hsieh JT, Chang CH, Lin JJM (2015) Direct kinetic measurement of the reaction of the simplest criegee intermediate with water vapor. Science 347:751–754. https://doi.org/10.1126/science.1261549
Chhantyal-Pun R, Davey A, De S et al (2015) A kinetic study of the CH2OO Criegee intermediate self-reaction, reaction with SO2 and unimolecular reaction using cavity ring-down spectroscopy. Phys Chem Chem Phys 17:3617–3626. https://doi.org/10.1039/c4cp04198d
Novelli A, Hens K, Ernest CT et al (2017) estimating the atmospheric concentration of Criegee intermediates and their possible interference in a FAGe-LIF instrument. Atmos Chem Phys 17:7807–7826. https://doi.org/10.5194/acp-17-7807-2017
Berndt T, Jokinen T, Sipilä M et al (2014) H2SO4 formation from the gas-phase reaction of stabilized Criegee Intermediates with SO2: influence of water vapour content and temperature. Atmos Environ 89:603–612. https://doi.org/10.1016/j.atmosenv.2014.02.062
Mauldin RL, Berndt T, Sipilä M et al (2012) A new atmospherically relevant oxidant of sulphur dioxide. Nature 488:193–196. https://doi.org/10.1038/nature11278
Welz O, Eskola AJ, Sheps L et al (2014) Rate coefficients of C1 and C2 criegee intermediate reactions with formic and acetic acid near the collision limit: direct kinetics measurements and atmospheric implications. Angew Chemie Int ed 53:4547–4550. https://doi.org/10.1002/anie.201400964
Vereecken L, Harder H, Novelli A (2012) The reaction of Criegee intermediates with NO, RO2, and SO2, and their fate in the atmosphere. Phys Chem Chem Phys 14:14682–14695. https://doi.org/10.1039/c2cp42300f
Chhantyal-Pun R, McGillen MR, Beames JM et al (2017) Temperature-dependence of the rates of reaction of trifluoroacetic acid with criegee intermediates. Angew Chemie Int ed 56:9044–9047. https://doi.org/10.1002/anie.201703700
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals (T. Theor Chem Acc 119:525
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Acc Chem Res 41:157–167. https://doi.org/10.1021/ar700111a
Dash MR, Muthiah B, Mishra SS et al (2021) Kinetic insights into ethynyl radical with isobutane and neopentane. Theor Chem Acc 140:1–9. https://doi.org/10.1007/s00214-021-02833-x
Arathala P, Musah RA (2022) Theoretical study of the atmospheric chemistry of methane sulfonamide initiated by OH radicals and the CH3S(O)2N•H + 3O2 reaction. J Phys Chem A 126:9447–9460. https://doi.org/10.1021/acs.jpca.2c06432
Ali MA, Dash MR, Al Maieli LM (2022) Catalytic effect of CO2 and H2O Molecules on • CH3 +3O2 Reaction. Catalysts. https://doi.org/10.3390/catal12070699
Lily M, Baidya B, Wang W et al (2020) Atmospheric chemistry of CHF2CF2OCH2CF3: reactions with Cl atoms, fate of CHF2CF2OC•HCF3 radical, formation of OH radical and Criegee Intermediate. Atmos Environ 242:117805. https://doi.org/10.1016/j.atmosenv.2020.117805
Liu B, Zhou Z, Zhang Z, Ning H (2022) Theoretical study on abstraction and addition reaction kinetics for a medium-size unsaturated methyl ester: methyl-3-hexenoate + H/OH radicals. J Phys Chem A 126:9461–9474. https://doi.org/10.1021/acs.jpca.2c06249
Balaganesh M, Dash MR, Rajakumar B (2014) Experimental and computational investigation on the gas phase reaction of ethyl formate with Cl atoms. J Phys Chem A 118:5272–5278. https://doi.org/10.1021/jp502963w
Dash MR, Balaganesh M, Rajakumar B (2014) Rate coefficients for the gas-phase reaction of OH radical with α-pinene: an experimental and computational study. Mol Phys 112:1495–1511. https://doi.org/10.1080/00268976.2013.840395
Dash MR, Rajakumar B (2015) Abstraction and addition kinetics of C2H radicals with CH4, C2H6, C3H8, C2H4, and C3H6: CVT/SCT/ISPe and hybrid meta-DFT methods. Phys Chem Chem Phys 17:3142–3156. https://doi.org/10.1039/c4cp04677c
Dash MR, Mishra SS (2022) Mechanistic and kinetic approach on methyl isocyanate (CH3NCO) with OH and Cl. Mol Phys. https://doi.org/10.1080/00268976.2022.2124933
Dash MR, Mishra SS (2021) Theoretical kinetic studies of ethynyl radical with n-butane. J Phys Org Chem 34:1–8. https://doi.org/10.1002/poc.4249
Dash MR, Rajakumar B (2013) experimental and theoretical rate coefficients for the gas phase reaction of β-Pinene with OH radical. Atmos Environ 79:161–171. https://doi.org/10.1016/j.atmosenv.2013.05.039
Noga J, Bartlett RJ (1986) The full CCSDT model for molecular electronic structure. J Chem Phys 86:7041–7050. https://doi.org/10.1063/1.452353
Rienstra-Kiracofe JC, Allen WD, Schaefer HF (2000) C2H5+O2 reaction mechanism: high-level ab initio characterizations. J Phys Chem A 104:9823–9840. https://doi.org/10.1021/jp001041k
Galano A, Muñoz-Rugeles L, Alvarez-Idaboy JR et al (2016) Hydrogen abstraction reactions from phenolic compounds by peroxyl radicals: multireference character and density functional theory rate constants. J Phys Chem A 120:4634–4642. https://doi.org/10.1021/acs.jpca.5b07662
Bai FY, Zhu XL, Jia ZM et al (2015) Theoretical studies of the reactions CFxH3−xCOOR+Cl and CF3COOCH3+OH. ChemPhysChem 16:1768–1776. https://doi.org/10.1002/cphc.201402799
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision B.01. Gaussian 09, Revis. B.01. Gaussian Inc., Wallingford
Garrett BC, Truhlar DG (1979) Generalized transition state theory. Classical mechanical theory and applications to collinear reactions of hydrogen molecules. J Phys Chem 83:1052–1079. https://doi.org/10.1021/j100471a031
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) Improved treatment of threshold contributions in variational transition-state theory. J Phys Chem 84:1730–1748. https://doi.org/10.1021/j100450a013
Gonzalez-Lafont A, Truong TN, Truhlar DG (1991) Interpolated variational transition-state theory: practical methods for estimating variational transition-state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J Chem Phys 95:8875–8894. https://doi.org/10.1063/1.461221
Lu DH, Truong TN, Melissas VS et al (1992) POLYRATe 4: a new version of a computer program for the calculation of chemical reaction rates for polyatomics. Comput Phys Commun 71:235–262. https://doi.org/10.1016/0010-4655(92)90012-N
Liu YP, Lynch GC, Truong TN et al (1993) Molecular modeling of the kinetic isotope effect for the [1,5] sigmatropic rearrangement of cis-1,3-pentadiene. J Am Chem Soc 115:2408–2415. https://doi.org/10.1021/ja00059a041
Chuang YY, Corchado JC, Truhlar DG (1999) Mapped interpolation scheme for single-point energy corrections in reaction rate calculations and a critical evaluation of dual-level reaction path dynamics methods. J Phys Chem A 103:1140–1149. https://doi.org/10.1021/jp9842493
Canneaux S, Bohr F, Henon E (2014) KiSThelP: a program to predict thermodynamic properties and rate constants from quantum chemistry results. J Comput Chem 35:82–93. https://doi.org/10.1002/jcc.23470
Wei WM, Yang X, Zheng RH et al (2015) Theoretical studies on the reactions of the simplest Criegee intermediate CH2OO with CH3CHO. Comput Theor Chem 1074:142–149. https://doi.org/10.1016/j.comptc.2015.10.013
Kaipara R, Rajakumar B (2018) Temperature-dependent kinetics of the reaction of a criegee intermediate with propionaldehyde: a computational investigation. J Phys Chem A 122:8433–8445. https://doi.org/10.1021/acs.jpca.8b06603
Crehuet R, Anglada JM, Cremer D, Bofill JM (2002) Reaction modes of carbonyl oxide, dioxirane, and methylenebis(oxy) with ethylene: a new reaction mechanism. J Phys Chem A 106:3917–3929. https://doi.org/10.1021/jp0142031
Dash MR, Srinivasulu G, Rajakumar B (2015) Experimental and computational investigation on the gas phase reaction of p-cymene with Cl atoms. J Phys Chem A 119:559–570. https://doi.org/10.1021/jp509800g
Zheng J, Zhang S, Lynch BJ, Corchado JC, Chuang YY, Hu WPY, Liu P, Lynch GC, Nguyen KA, Jackels CFR, Ellingson BA, Melissas VS, Villà J, Rossi CEL, Pu J, Albu TV, Steckler R, Garrett BC, IADTDG (2008) POLYRATE, version 2008. University of Minnesota, Minneapolis
Zheng J, Zhang S, Corchado JC, Chuang Y-Y, Coitiño EL E, BA TD (2010) GAUSSRATE, version 2009-A University of Minnesota, Minneapolis
Jalan A, Allen JW, Green WH (2013) Chemically activated formation of organic acids in reactions of the Criegee intermediate with aldehydes and ketones. Phys Chem Chem Phys. https://doi.org/10.1039/c3cp52598h
Hippler H, Troe J, Wendelken HJ (1983) Collisional deactivation of vibrationally highly excited polyatomic molecules. II. Direct observations for excited toluene. J Chem Phys 78:6709–6717. https://doi.org/10.1063/1.444670
Saheb V (2021) Detailed theoretical kinetics studies on the product formation from the reaction of the criegee intermediate CH2OO with H2O molecule. Theor Chem Acc. https://doi.org/10.1007/s00214-021-02779-0
Acknowledgements
The authors thank Department of Chemistry, National Taiwan University, Taipei, Taiwan, for providing computer facilities. This work was supported through the seed funding grant by the Dean Research & consultancy of DIT University, Dehradun, India [Project No. DITU/R & D/2022/015/Chemistry]. The authors thank Professor Donald G. Truhlar and his group for providing the POLYRATE 2008 and GAUSSRATE 2009A programs.
Author information
Authors and Affiliations
Contributions
MRD and BM completed all the electronic structure calculations, and BM completed the chemical kinetic calculations. MRD and SSM did all the analysis and interpretation of data. MRD and SSM prepared the draft of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was stated by the author(s).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dash, M.R., Muthiah, B. & Mishra, S.S. Formation of alkoxymethyl hydroperoxides and alkyl formates from simplest Criegee intermediate (CH2OO) + ROH (R=CH3, CH3CH2, and (CH3)2CH) reaction systems. Theor Chem Acc 143, 29 (2024). https://doi.org/10.1007/s00214-024-03104-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03104-1