Abstract
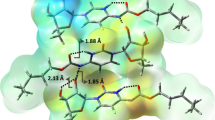
Cancer is one of the disorders that causes the most fatalities in modern times and is responsible for a significant number of deaths each year. The accurate administration of this disease has been hampered by a variety of factors including variances in the disease found in various regions of the globe. Several medications, in addition to diagnostic procedures, have evolved as a result of advancements in scientific research in order to treat certain cancers and have contributed to the cure of this disease to some degree. The study of the medical repercussions of cancer remains an intriguing and important subject of interest even after all the efforts that have been accomplished over a long period of scientific research. The purpose of this work was to contribute to the advancement of such fundamental scientific research by describing the attribution of unique modes of vibration of the antiemetic aprepitant as well as the anticancer medication capecitabine. Using standard FT-IR spectrum analysis and molecular docking techniques, the exact atomic-level mechanism of aprepitant and capecitabine is determined, by targeting the human NK1 substance P receptor (Human brain NK1 receptor) and thymidine phosphatase (cancer tissue) proteins. The quantum mechanical wavelength–energy relation was used to anticipate the associated energy intervals in order to assess the bioactivity of the molecules and the transfer of charge between HOMO and LUMO orbitals. Molecular docking investigations of aprepitant and capecitabine with the NK1 receptor and the thymidine phosphorylase proteins have been determined, respectively. The root mean square deviation and binding energy, as well as the types of bonds formed and the exact pathway taken by the anticancer medications in their interaction with the receptor, are established. This knowledge may be used to improve the mechanism of action, efficacy, and effectiveness of NK1 receptor antagonist drugs and anticancer medication, as well as to design innovative anticancer agents.










Similar content being viewed by others
Availability of data and materials
Data will be made available on request.
References
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/CAAC.21660
“Worldwide cancer statistics | Cancer research UK.” https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-Zero (Accessed Jan. 06, 2023)
Stocks M (2013) The small molecule drug discovery process – from target selection to candidate selection. Introd Biol Small Mol Drug Res Dev Theory Case Stud. https://doi.org/10.1016/B978-0-12-397176-0.00003-0
“Aprepitant.” Meyler’s Side Eff. Drugs 2016, https://doi.org/10.1016/B978-0-444-53717-1.00022-6
Ruby C (2013) Aprepitant. xPharm Compr Pharmacol Ref. https://doi.org/10.1016/B978-008055232-3.63742-0
House L, Ramirez J, Seminerio M, Mirkov S, Ratain MJ (2015) In vitro glucuronidation of aprepitant: a moderate inhibitor of UGT2B7. Xenobiotica 45(11):990–998. https://doi.org/10.3109/00498254.2015.1038743
Ruby C (2010) Aprepitant. xPharm Compr Pharmacol Ref. https://doi.org/10.1016/B978-008055232-3.63742-0
Jordan K, Feyer PC, Ortner P (2011) Prophylaxis and treatment of chemotherapy-induced nausea and vomiting. Support Oncol. https://doi.org/10.1016/B978-1-4377-1015-1.00003-5
Wu YJ (2012) Heterocycles and medicine: a survey of the heterocyclic drugs approved by the U.S. FDA from 2000 to present. Prog Heterocycl Chem 24:1–53. https://doi.org/10.1016/B978-0-08-096807-0.00001-4
Rider BJ (2007) Capecitabine. xPharm Compr Pharmacol Ref. https://doi.org/10.1016/B978-008055232-3.62967-8
Janney LM, Waterbury NV (2005) Capecitabine-warfarin interaction. Ann Pharmacother 39(9):1546–1551. https://doi.org/10.1345/APH.1G153
“Melphalan | 148–82–3.” https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2492687.htm (Accessed Oct. 25, 2021)
K R Cousins (2011)“Computer review of chemdraw ultra 120”. J Am Chem Soc 133(21): 8388 https://doi.org/10.1021/JA204075S
Simmons MA (2010) Tachykinin receptor agents. xPharm Compr Pharmacol Ref. https://doi.org/10.1016/B978-008055232-3.63832-2
Welston K, May D (2017) Gastrointestinal drugs. Side Eff Drugs Annu 39:373–387. https://doi.org/10.1016/BS.SEDA.2017.06.022
Mader MM, Henry JR (2007) Antimetabolites. Compr Med Chem II:55–79. https://doi.org/10.1016/B0-08-045044-X/00204-2
McMillin GA, Wadelius M, Pratt VM (2018) Pharmacogenetics. Princ Appl Mol Diagnostics. https://doi.org/10.1016/B978-0-12-816061-9.00011-4
Wyatt MD, Wilson DM (2009) Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci 66(5):788–799. https://doi.org/10.1007/S00018-008-8557-5
“UV/Vis/NIR Spectrophotometers and accessories | PerkinElmer.” https://www.perkinelmer.com/uk/category/uv-vis-spectroscopy-uv (Accessed Oct. 25, 2021)
“IR spectrum table.” https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (Accessed Oct. 25, 2021)
“IR table.” https://www.chem.ucla.edu/~bacher/General/30BL/IR/ir.html (Accessed Oct. 25, 2021)
Ramana PV et al (2022) Spectroscopic, quantum mechanical, electronic excitation properties (Ethanol solvent), DFT investigations and molecular docking analysis of an anti-cancer drug Bendamustine. J Mol Struct 1253:132211. https://doi.org/10.1016/j.molstruc.2021.132211
Mariappan G, Sundaraganesan N, Manoharan S (2012) The spectroscopic properties of anticancer drug Apigenin investigated by using DFT calculations, FT-IR, FT-Raman and NMR analysis. Spectrochim Acta Part A Mol Biomol Spectrosc 1(95):86–99. https://doi.org/10.1016/j.saa.2012.04.089
“Infrared spectra: identifying functional groups.” https://sites.science.oregonstate.edu/~gablek/CH335/Chapter10/IR.htm (Accessed Oct. 25, 2021)
Joseph L, Sajan D, Chaitanya K, Devarajegowda HC, Isac J (2013) Density functional theory study, FT-iR and FT-Raman spectra and SQM force field calculation for vibrational analysis of 1, 3-Bis (hydroxymethyl) benzimidazolin-2-one. Spectrochim Acta Part A Mol Biomol Spectrosc 1(114):432–440. https://doi.org/10.1016/j.saa.2013.05.003
Nagib DA, Macmillan DWC (2011) Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 480(7376):224–228. https://doi.org/10.1038/nature10647
Mary YS, Panicker CY, Anto PL, Sapnakumari M, Narayana B, Sarojini BK (2015) Molecular structure, FT-IR, NBO, HOMO and LUMO, MEP and first order hyperpolarizability of (2E)-1-(2, 4-dichlorophenyl)-3-(3, 4, 5-trimethoxyphenyl) prop-2-en-1-one by HF and density functional methods. Spectrochim Acta Part A Mol Biomol Spectrosc 25(135):81–92. https://doi.org/10.1016/J.SAA.2014.06.140
Sertbakan TR (2017) Structure, spectroscopic and quantum chemical investigations of 4-Amino-2-Methyl-8-(Trifluoromethyl)quinoline. Celal Bayar Üniv Fen Bilim Derg 13(4):851–861. https://doi.org/10.18466/cbayarfbe.339858
Sri NU, Chaitanya K, Prasad MV, Veeraiah V, Veeraiah A (2012) Experimental (FT-IR, FT-Raman and UV–Vis spectra) and density functional theory calculations of diethyl 1H-pyrazole-3, 5-dicarboxylate. J Mol Struct 18(1019):68–79. https://doi.org/10.1016/j.molstruc.2012.03.032
Compounds O (1964) CS stretching frequency. Science 42(80):36–42
Prasad KV, Muthu S, Santhamma C (2017) Spectroscopic (FT-IR, FT-Raman, UV–Visible) and quantum chemical studies of 4-Chloro-3-iodobenzophenone. J Mol Struct 15(1128):685–693. https://doi.org/10.1016/J.MOLSTRUC.2016.09.037
Liu J et al (2015) Characterization and pharmacokinetic study of aprepitant solid dispersions with soluplus®. Molecules 20(6):11345–11356. https://doi.org/10.3390/molecules200611345
Helmy R et al (2003) Characterization and quantitation of aprepitant drug substance polymorphs by attenuated total reflectance Fourier transform infrared spectroscopy. Anal Chem 75(3):605–611. https://doi.org/10.1021/ac020538i
Atkins M, López IG, Kuo B (2022) Nausea and vomiting. Compr Pharmacol. https://doi.org/10.1016/B978-0-12-820472-6.00182-1
Prasad KV, Samatha K, Rao DJ, Santhamma C, Muthu S, Heron BM (2015) Spectroscopic studies (FT-IR, FT-Raman, UV–Visible), normal co-ordinate analysis, first-order hyperpolarizability and HOMO, LUMO studies of 3, 4-dichlorobenzophenone by using density functional methods. Spectrochim Acta Part A Mol Biomol Spectrosc 5(151):644–654. https://doi.org/10.1016/j.saa.2015.07.001
Mary YS, Jojo PJ, Panicker CY, Van Alsenoy C, Ataei S, Yildiz I (2014) “Theoretical investigations on the molecular structure, vibrational spectra, HOMO–LUMO and NBO analysis of 5-chloro-2-((4-chlorophenoxy)methyl)benzimidazole.” Spectrochim Acta Part A Mol Biomol Spectrosc 122:499–511. https://doi.org/10.1016/J.SAA.2013.11.025
Mary YS, Jojo PJ, Panicker CY, Van Alsenoy C, Ataei S, Yildiz I (2014) Quantum mechanical and spectroscopic (FT-IR, FT-Raman, 1H NMR and UV) investigations of 2-(phenoxy methyl)benzimidazole. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/J.SAA.2014.01.068
“Aprepitant,” Meyler’s Side Eff Drugs, p 657, 2016, https://doi.org/10.1016/B978-0-444-53717-1.00022-6
“Aprepitant: uses, interactions, mechanism of action | drugbank online.” https://go.drugbank.com/drugs/DB00673 (Accessed Jun. 18, 2022)
Dolan RD, Rohani TS, Muttineni D, Mashimo H (2022) The physiology and pharmacology of diabetic gastropathy management. Compr Pharmacol. https://doi.org/10.1016/B978-0-12-820472-6.00045-1
Prasad KV, Ramana PV (2023) Vibrational spectroscopic studies, DFT, and molecular docking investigations of 4-fluoro-3-methyl benzophenone. Vib Spectrosc 1(126):103532. https://doi.org/10.1016/j.vibspec.2023.103532
Beauchamp spectroscopy tables 1. https://edisciplinas.usp.br/pluginfile.php/6971133/mod_resource/content/1/spec_ir_nmr_spectra_tables.pdf
Alen S, Sajan D, Joseph L, Chaitanya K, Shettigar V, Jothy VB (2015) Synthesis, growth, vibrational spectral investigations and structure-property relationship of an organic NLO crystal: 3,4-dimethoxy chalcone. Chem Phys Lett 636:208–215. https://doi.org/10.1016/j.cplett.2015.07.030
Ramana PV, Krishna YR, Mouli KC (2022) Experimental FT-IR and UV–Vis spectroscopic studies and molecular docking analysis of anti-cancer drugs exemestane and Pazopanib. J Mol Struct 5(1263):133051. https://doi.org/10.1016/j.molstruc.2022.133051
Yahyaei B, Pourali P (2019) One step conjugation of some chemotherapeutic drugs to the biologically produced gold nanoparticles and assessment of their anticancer effects. Sci Rep 9(1):1–16. https://doi.org/10.1038/s41598-019-46602-0
Martino R, Gilard V, Malet-Martino M (2008) Fluorine-19 or Phosphorus-31 NMR spectroscopy: a powerful technique for biofluid metabolic studies and pharmaceutical formulation analysis of fluorinated or phosphorylated drugs. NMR Spectrosc Pharm Anal. https://doi.org/10.1016/B978-0-444-53173-5.00015-9
Vardanyan R, Hruby V (2016) Antineoplastic agents. Synth Best-Seller Drugs. https://doi.org/10.1016/B978-0-12-411492-0.00028-6
“Antineoplastic agents”. Cancer Bull 27(5): 80–82, 1975, https://doi.org/10.1016/B978-0-12-411492-0.00028-6
Reddy VP (2015) Organofluorine compounds as anticancer agents. Organofluor Compd Biol Med. https://doi.org/10.1016/B978-0-444-53748-5.00009-5
Shrivastava N, Iqbal B, Ali J, Baboota S (2021) Chemopreventive and therapeutic potential of natural agents and their combinations for breast cancer. Discov Dev Anti-Breast Cancer Agents from Nat Prod. https://doi.org/10.1016/B978-0-12-821277-6.00009-X
“Everolimus: uses, interactions, mechanism of action | drugbank online.” https://go.drugbank.com/drugs/DB01590 (Accessed Oct. 25, 2021)
Balaev VV, Prokofev II, Gabdoulkhakov AG, Betzel C, Lashkov AA (2018) Crystal structure of pyrimidine-nucleoside phosphorylase from Bacillus subtilis in complex with imidazole and sulfate. Acta Crystallogr Sect F: Struct Biol Commun 74(4):193–197. https://doi.org/10.1107/S2053230X18002935
Adeniji SE, Uba S, Uzairu A (2019) Molecular docking study for evaluating the binding mode and interaction of 2, 4-disubstituted quinoline and its derivatives as potent anti-tubercular agents against Lipoate protein B (LipB). Turk Comput Theor Chem 3(1):17–24. https://doi.org/10.33435/tcandtc.458615
Jones D et al (2021) Improved protein-ligand binding affinity prediction with structure-based deep fusion inference. J Chem Inf Model 61(4):1583–1592. https://doi.org/10.1021/acs.jcim.0c01306
Gurung AB, Bhattacharjee A, Ali MA (2016) Exploring the physicochemical profile and the binding patterns of selected novel anticancer Himalayan plant-derived active compounds with macromolecular targets. Inform Med Unlocked 5(July):1–14. https://doi.org/10.1016/j.imu.2016.09.004
Raajaraman BR, Sheela NR, Muthu S (2019) Spectroscopic, quantum computational and molecular docking studies on 1-phenylcyclopentane carboxylic acid. Comput Biol Chem 82:44–56. https://doi.org/10.1016/J.COMPBIOLCHEM.2019.05.011
Lawley PD, Phillips DH (1996) DNA adducts from chemotherapeutic agents. Mutat Res—Fundam Mol Mech Mutagen 355(1–2):13–40. https://doi.org/10.1016/0027-5107(96)00020-6
Povirk LF, Shuker DE (1994) DNA damage and mutagenesis induced by nitrogen mustards. Mutat Res Genet Toxicol 318(3):205–226. https://doi.org/10.1016/0165-1110(94)90015-9
“RCSB PDB - 4X46: X-RAY structure thymidine phosphorylase from Salmonella typhimurium complex with SO4 at 2.19 A.” https://www.rcsb.org/structure/4X46 (Accessed Jun. 18, 2022)
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/JCC.21334
“Free download: BIOVIA discovery studio visualizer - dassault systèmes.” https://discover.3ds.com/discovery-studio-visualizer-download (Accessed Sep. 06, 2021)
Ramana PV, Krishna YR, Mouli KC (2022) Experimental ( FT-IR, UV-Vis ) spectroscopic analysis and molecular docking investigations of anti-cancer drugs Alkeran and Bicalutamide. J Mol Struct 1270:133984. https://doi.org/10.1016/j.molstruc.2022.133984
Malińska M et al (2014) Crystal structure and tautomerism of capecitabine. J Pharm Sci 103(2):587–593. https://doi.org/10.1002/jps.23831
Funding
The program is self-financed.
Author information
Authors and Affiliations
Contributions
P. VR contributed to the conceptualization, methodology, investigation, validation, software, formal analysis, writing—original draft, writing—editing, visualization, supervision, project administration and resources.
Corresponding author
Ethics declarations
Competing interests
I disregard conflicting interests.
Ethical approval
This study does not have any clinical trials on humans and no experiments have been conducted on any animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramana, P.V. Experimental spectroscopic and molecular docking investigations of the anticancer drugs aprepitant and capecitabine. Theor Chem Acc 142, 114 (2023). https://doi.org/10.1007/s00214-023-03055-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03055-z