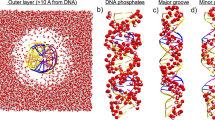
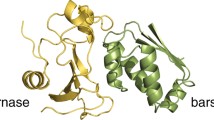
Abstract
Molecular dynamics simulations have been carried out to explore the effect of complex formation between the N-terminal domain of the \(\lambda \)-repressor protein (in dimeric form) and the corresponding DNA operator on low-frequency intermolecular vibrational modes of water confined at the interface. The calculations demonstrated enhanced back-scattering of interfacial water due to increased caging effects, the effect being greater for water molecules that are associated with direct binding process. Highest degree of caging effect has been identified with the water molecules that are engaged in forming hydrogen-bonded bridges either near directly bound residues or in establishing contacts between the unbound DNA and protein residues. This leads to blue shifts of the O\(\cdots \)O\(\cdots \)O bending mode of water and the effect is maximum for the bridged water. The analyses further demonstrated that the local randomness of the interfacial water molecules strongly depends on the conformational rigidity of the DNA and the protein components.





Similar content being viewed by others
Data Availability
All data used in this study are openly available with contacting Sandip Mondal
References
Nelson DL, Cox MM (2000) Lehninger Principles of Biochemistry. Worth, New York
Branzei D, Foiani M (2008) Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol 9(4):297–308
Beamer LJ, Pabo CO (1992) Refined 1.8 å crystal structure of the \(\lambda \)-repressor-operator complex. J Mol Biol 227(1):177–196
Pavletich NP, Pabo CO (1993) Crystal structure of a five-finger gli-dna complex: new perspectives on zinc fingers. Science 261:1701–1707
Hamès C et al (2008) Structural basis for leafy floral switch function and similarity with helix-turn-helix proteins. EMBO J. 27(19):2628–2637
Watkins D, Mohan S, Koudelka GB, Williams LD (2010) Sequence recognition of DNA by protein-induced conformational transitions. J Mol Biol 396(4):1145–1164
Wolberger C, Dong Y, Ptashne M, Harrison SC (1988) Structure of a phage 434 cro/DNA complex. Nature 335:789–795
Esadze A, Iwahara J (2014) Stopped-flow fluorescence kinetic study of protein sliding and intersegment transfer in the get DNA search process. J Mol Biol 426(1):230–244
Batabyal S, Choudhury S, Sao D, Mondol T, Pal SK (2014) Dynamical perspective of protein-DNA interaction. Biomolecular concepts 5(1):21–43
Samiee KT, Foquet M, Guo L, Cox E, Craighead H (2005) \(\lambda \)-repressor oligomerization kinetics at high concentrations using fluorescence correlation spectroscopy in zero-mode waveguides. Biophys J 88(3):2145–2153
Levy Y, Onuchic JN (2006) Water mediation in protein folding and molecular recognition. Annu Rev Biophys Biomol Struct 35:389–415
Yamane T, Okamura H, Ikeguchi M, Nishimura Y, Kidera A (2008) Water-mediated interactions between DNAand phob dna-binding/transactivation domain: Nmr-restrained molecular dynamics in explicit water environment. Proteins: Struct Funct Bioinf 71(4):1970–1983
Qin Y et al (2013) Direct probing of solvent accessibility and mobility at the binding interface of polymerase (dpo4)-dna complex. J Phy Chem A 117(50):13926–13934
Balaeff A, Churchill M. E, Schulten K (1998) Structure prediction of a complex between the chromosomal protein hmg-d and dna. Proteins: Struct, Funct, Bioinf 30(2):113–135
Xhani S, Esaki S, Huang K, Erlitzki N, Poon GM (2017) Distinct roles for interfacial hydration in site-specificDNA recognition by ets-family transcription factors. J Phys Chem B 121(13):2748–2758
Etheve L, Martin J, Lavery R (2016) Dynamics and recognition within a protein-dna complex: a molecular dynamics study of the skn-1/dna interaction. Nucleic Acids Res 44(3):1440–1448
Song W, Guo J-T (2015) Investigation of arc repressor dna-binding specificity by comparative molecular dynamics simulations. J Biomol Struct Dyn 33(10):2083–2093
Yamasaki S, Terada T, Kono H, Shimizu K, Sarai A (2012) A new method for evaluating the specificity of indirect readout in protein-dna recognition. Nucleic Acids Res 40(17):e129–e129
Sinha SK, Bandyopadhyay S (2011) Dynamic properties of water around a protein-dna complex from molecular dynamics simulations. J Chem Phys 135(13):135101
Lenz SA, Wetmore SD (2016) Evaluating the substrate selectivity of alkyladenine dna glycosylase: the synergistic interplay of active site flexibility and water reorganization. Biochemistry 55(5):798–808
Mondal S, Chakraborty K, Bandyopadhyay S (2017) Microscopic understanding of the conformational features of a protein-dna complex. Phys Chem Chem Phys 19(48):32459–32472
Mondal S, Bandyopadhyay S (2019) Flexibility of the binding regions of a protein-dna complex and the structure and ordering of interfacial water. J Chem Inf Model 59(10):4427–4437
Mondal S, Bandyopadhyay S (2020) Heterogeneous dynamical environment at the interface of a protein-dna complex. Langmuir 36(17):4567–4581
Pabo CO, Lewis M (1982) The operator-binding domain of \(\lambda \)-repressor: Structure and dna recognition. Nature 298:443–447
Details of the repressor-operator interactions (1988) Jordan, S. R. & Pabo, C. O. Structure of the lambda complex at 2.5 å resolution. Science 242:893–899
Phillips JC et al (2005) Scalable molecular dynamics with namd. J Comput Chem 26(16):1781–1802
Foloppe N, MacKerell AD Jr (2000) All-atom empirical force field for nucleic acids: I. parameter optimization based on small molecule and condensed phase macromolecular target data. J Comput Chem 21(2):86–104
Mackerell AD, Banavali NK (2000) All-atom empirical force field for nucleic acids: Ii. application to molecular dynamics simulations of dna and rna in solution. J Comput Chem 21(2):105–120
MacKerell AD Jr et al (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102(18):3586–3616
Mackerell AD Jr (2004) Empirical force fields for biological macromolecules: overview and issues. J Comput Chem 25(13):1584–1604
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Ryckaert J-P, Ciccotti G, Berendsen HJ (1977) Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 23(3):327–341
Allen MP, Tildesley DJ (1987) Computer Simulation of Liquids (Oxford university press,)
Darden T, York D, Pedersen L (1993) Particle mesh ewald: An n-log(n) method for ewald sums in large systems. J Chem Phys 98(12):10089–10092
Walrafen G, Chu Y (1995) Linearity between structural correlation length and correlated-proton raman intensity from amorphous ice and supercooled water up to dense supercritical steam. J Phys Chem 99(28):11225–11229
Walrafen G, Chu Y, Piermarini G (1996) Low-frequency raman scattering from water at high pressures and high temperatures. J Phys Chem 100(24):10363–10372
Brubach J-B, Mermet A, Filabozzi A, Gerschel A, Roy P (2005) Signatures of the hydrogen bonding in the infrared bands of water. J Chem Phys 122(18):184509
Li J (1996) Inelastic neutron scattering studies of hydrogen bonding in ices. J Chem Phys 105(16):6733–6755
Crupi V, Dianoux AJ, Majolino D, Migliardo P, Venuti V (2002) Dynamical response of liquid water in confined geometry by laser and neutron spectroscopies. Phys Chem Chem Phys 4(12):2768–2773
Martì J, Padró JÀ, Guàrdia E (1996) Molecular dynamics simulation of liquid water along the coexistence curve: hydrogen bonds and vibrational spectra. J Chem Phys 105(2):639–649
Padró JÀ, Martì J (2003) An interpretation of the low-frequency spectrum of liquid water. J Chem Phys 118(1):452–453
Svishchev I. M, Kusalik P. G (1994) Rotational dynamics in liquid water: a simulation study of librational motions. J Chem Soc, Faraday Trans 90(10):1405–1409
Mazur K, Heisler IA, Meech SR (2012) Water dynamics at protein interfaces: ultrafast optical kerr effect study. J Phys Chem A 116(11):2678–2685
Paciaroni A et al (2008) Fingerprints of amorphous icelike behavior in the vibrational density of states of protein hydration water. Phys Rev Lett 101(14):148104
Perticaroli S et al (2010) Broadband depolarized light scattering study of diluted protein aqueous solutions. J Phys Chem B 114(24):8262–8269
Luong TQ, Xu Y, Bründermann E, Leitner DM, Havenith M (2016) Hydrophobic collapse induces changes in the collective protein and hydration low frequency modes. Chem Phys Lett 651:1–7
Heyden M, Havenith M (2010) Combining thz spectroscopy and md simulations to study protein-hydration coupling. Methods 52(1):74–83
Wirtz H et al (2018) Hydrophobic collapse of ubiquitin generates rapid protein-water motions. Biochemistry 57(26):3650–3657
Shiraga K, Ogawa Y, Kondo N (2016) Hydrogen bond network of water around protein investigated with terahertz and infrared spectroscopy. Biophys J 111(12):2629–2641
Lin S-T, Blanco M, Goddard WA III (2003) The two-phase model for calculating thermodynamic properties of liquids from molecular dynamics: validation for the phase diagram of lennard-jones fluids. J Chem Phys 119(22):11792–11805
Acknowledgements
This study was supported by grant received from the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India (Ref. No. CRG/2020/000044) as well as grant received under DST-FIST programme. Sandip Mondal thanks University Grants Commission (UGC), Government of India (23/12/2012(ii)EU-V), while Krishna P. Ghanta and Souvik Mondal thank Council of Scientific and Industrial Research (CSIR), Government of India (09/081(1312)/2017-EMR-I, dated 18.10.2017 and 09/081(1272)/2015-EMR-I, dated 29.12.2015) for scholarships This work used the resources of the supercomputing facility of the Indian Institute of Technology Kharagpur established under National Supercomputing Mission (NSM), Government of India.
Author information
Authors and Affiliations
Contributions
SM performed the simulations and carried out partial analysis, KPG and SM carried out analysis and prepared the figures, SB conceptualized the idea of the manuscript. All the authors were involved in preparing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mondal, S., Ghanta, K.P., Mondal, S. et al. Vibrational spectrum and randomness of water at the interface of a protein–DNA complex. Theor Chem Acc 142, 75 (2023). https://doi.org/10.1007/s00214-023-03017-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03017-5