Abstract
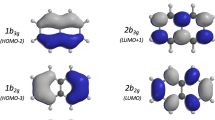
In an astrochemical and environmental context, this work constitutes a step forward in understanding the photo-reactivity of polycyclic aromatic hydrocarbons (PAHs) with water molecules and in water ice under irradiation with low energy photons. The role of charge transfer states \(\hbox {PAH}^+\hbox {-H}_2\hbox {O}^-\) has been proposed, motivating the study of the electronic excited states up to about 6 eV of planar and bowl-shaped PAHs, namely pyrene \(\hbox {C}_{16}\hbox {H}_{10}\) and corannulene \(\hbox {C}_{20}\hbox {H}_{10}\), interacting with water clusters of different sizes and orientations, using a time-dependent density functional theory approach. In the case of pyrene, the systematic occurrence of low energy excitations from \(\pi\) orbitals to diffuse orbitals located on some water molecules, mixed with the Rydberg orbitals (R/wat), was found. Such excitations are more numerous and possess larger oscillator strengths when (i) the number of water molecules increases up to representing a first layer of hexagonal water ice and (ii) for the arrangements leading to the lower vertical ionization potential values. In this case, the /wat orbitals are located on the most external H atoms and they may also mix with \(\pi ^{\star }\) orbitals. This accounts for the efficient reactivity of pyrene with water in water ice. In the case of corannulene, the main result is that, for the \(\hbox {C}_{20}\hbox {H}_{10}(\hbox {H}_{2}\hbox {O})_{3}\) isomer formed in a noble gas matrix, where \((\hbox {H}_{2}\hbox {O})_{3}\) interacts with the concave face of corannulene, no \(\pi \rightarrow \hbox {R/wat}\) transition is observed. It is in line with the lack of reactivity of corannulene with water in a noble gas matrix.









Similar content being viewed by others
References
Seinfeld JH, Pandis SN (2016) Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. John Wiley & Sons Inc., Hoboken, NJ
Vinklárek IS, Pysanenko A, Pluhařová E, Fárník M (2022) Uptake of hydrogen bonding molecules by benzene nanoparticles. J Phys Chem Lett 13:3781–3788
Ravishankara AR (1997) Heterogeneous and multiphase chemistry in the troposphere. Science 276(5315):1058–1065
George C, Ammann M, D’Anna B, Donaldson DJ, Nizkorodov SA (2015) Heterogeneous photochemistry in the atmosphere. Chem Rev 115(10):4218–4258
Allamandola LJ, Tielens AGGM, Barker JR (1985) Polycyclic aromatic-hydrocarbons and the unidentified infrared-emission bands—auto exhaust along the milky-way. Astrophys J 290(1):L25–L28
Léger A, Puget JL (1984) Identification of the unidentified IR emission features of interstellar dust? Astron Astrophys 137:L5–L8
Allamandola LJ, Tielens AG, Barker JR (1989) Interstellar polycyclic aromatic hydrocarbons: the infrared emission bands, the excitation/emission mechanism, and the astrophysical implications. Astrophys J Suppl Ser 71:733–775
Tielens A (2008) Interstellar polycyclic aromatic hydrocarbon molecules. Ann Rev Astron Astrophys 46(1):289–337
McGuire BA, Loomis RA, Burkhardt AM, Lee KLK, Shingledecker CN, Charnley SB, Cooke IR, Cordiner MA, Herbst E, Kalenskii S, Siebert MA, Willis ER, Xue C, Remijan AJ, McCarthy MC (2021) Detection of two interstellar polycyclic aromatic hydrocarbons via spectral matched filtering. Science 371:1265–1269
Bouwman J, Mattioda AL, Linnartz H, Allamandola LJ (2011) Photochemistry of pahs in cosmic water ice i. mid-ir spectroscopy and photoproducts. Astron Astrophys 525:A93
Noble JA, Michoulier E, Aupetit C, Mascetti J (2020) Influence of ice structure on the soft uv photochemistry of pahs embedded in solid water. Astron Astrophys 644:A22
Guennoun Z, Aupetit C, Mascetti J (2011) Photochemistry of coronene with water at 10 K: first tentative identification by infrared spectroscopy of oxygen containing coronene products. Phys Chem Chem Phys 13(16):7340–7347
Guennoun Z, Aupetit C, Mascetti J (2011) Photochemistry of pyrene with water at low temperature: study of atmospherical and astrochemical interest. J Phys Chem A 115:1844–1852
Noble JA, Jouvet C, Aupetit C, Moudens A, Mascetti J (2017) Efficient photochemistry of coronene:water complexes. Astron Astrophys 599:A124
Simon A, Noble JA, Rouaut G, Moudens A, Aupetit C, Iftner C, Mascetti J (2017) Formation of coronene: water complexes: Ftir study in argon matrices and theoretical characterisation. Phys Chem Chem Phys 19:8516–8529
Leboucher H, Mascetti J, Aupetit C, Noble JA, Simon A (2022) Water clusters in interaction with corannulene in a rare gas matrix: structures, stability and IR spectra. Photochem 2:237–262
Pérez C, Steber AL, Rijs AM, Temelso B, Shields GC, Lopez JC, Kisiel Z, Schnell M (2017) Corannulene and its complex with water: a tiny cup of water. Phys Chem Chem Phys 19:14214–14223
Lemmens AK, Gruet S, Steber AL, Antony J, Grimme S, Schnell M, Rijs AM (2019) Far-ir and uv spectral signatures of controlled complexation and microhydration of the polycyclic aromatic hydrocarbon acenaphthene. Phys Chem Chem Phys 21:3414–3422
Loru D, Steber AL, Pinacho P, Gruet S, Temelso B, Rijs AM, Perez C, Schnell M (2021) How does the composition of a pah influence its microsolvation? a rotational spectroscopy study of the phenanthrene-water and phenanthridine-water clusters. Phys Chem Chem Phys 23:9721–9732
Bouwman J, Paardekooper DM, Cuppen HM, Linnartz H, Allamandola LJ (2009) Real-time optical spectroscopy of vacuum ultraviolet irradiated pyrene:h2o interstellar ice. Astrophys J 700(1):56
Gudipati M, Allamandola L (2004) Polycyclic aromatic hydrocarbon ionization energy lowering in water ices. Astrophys J 615:L177–L180
Woon D, Park J (2004) Photoionization of benzene and small polycyclic aromatic hydrocarbons in ultraviolet-processed astrophysical ices: A computational study. Astrophys J 607:342–345
Gudipati M, Allamandola L (2006) Unusual stability of polycyclic aromatic hydrocarbon radical cations in amorphous water ices up to 120 K: Astronomical implications. Astrophys J 638:286–292
Michoulier E, Ben Amor N, Rapacioli M, Noble JA, Mascetti J, Toubin C, Simon A (2018) Theoretical determination of adsorption and ionisation energies of polycyclic aromatic hydrocarbons on water ice. Phys Chem Chem Phys 20(17):11941–11953
Novakovskaya YV, Stepanov NF (2004) Nonempirical description of the atmospherically important anionic species. i. water cluster anions. Struct Chem 15:65–70
Ben Amor N, Michoulier E, Simon A (2021) Electronic excited states of benzene in interaction with water clusters: influence of structure and size: Time dependent density functional theory vs multireference wavefunction approaches. Theo. Chem. Acc. 140:70
Finley J, Malmqvist P-Å, Roos BO, Serrano-Andrés L (1998) The multi-state CASPT2 method. Chem Phys Lett 288:299–306
Iftner C, Simon A, Korchagina K, Rapacioli M, Spiegelman F (2014) A density functional tight binding/force field approach to the interaction of molecules with rare gas clusters: Application to (c6h6)\(^{+/0}\)ar\(_n\) clusters. J Chem Phys 140:3
Simon A, Iftner C, Mascetti J, Spiegelman F (2015) Water clusters in an argon matrix: Infrared spectra from molecular dynamics simulations with a self-consistent charge density functional-based tight binding/force-field potential. J Phys Chem A 119:2449–2467
Noble JA, Aupetit C, Descamps D, Petit S, Simon A, Mascetti J, Ben Amor N, Blanchet V (2019) Ultrafast electronic relaxations from the S \(_{\rm 3}\) state of pyrene. Phys Chem Chem Phys 21(26):14111–14125
Dunning TH, Hay PJ (1977) Gaussian basis sets for molecular calculations. In: Schaefer F (ed) Methods of Electronic Structure Theory (H. Springer, US, pp 1–27
P.C̆àrsky P, Urban M (1980) Ab Initio Calculations: Methods and Applications in Chemistry, vol. 16 of Lecture Notes in Chemistry. Springer Berlin Heidelberg
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3–21+G basis set for first-row elements, Li-F. J Comput Chem 4(3):294–301
Widmark P-O, Malmqvist P-Å, Roos BO (1990) Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions: I. first row atoms. Theor. Chim. Acta 77(5):291–306
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theo. Chem. Acc. 120(1):215–241
Chai J-D, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:084106
Roos BO, Taylor PR, Siegbahn PE (1980) A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem Phys 48:157–173
Olsen J, Roos BO, Jørgensen P, Jensen HJA (1988) Determinant based configuration interaction algorithms for complete and restricted configuration interaction spaces. J Chem Phys 89:2185–2192
Malmqvist P-Å, Rendell A, Roos BO (1990) The restricted active space self-consistent-field method, implemented with a split graph unitary group approach. J Phys Chem 94:5477–5482
Malmqvist P-Å, Pierloot K, Shahi ARM, Cramer CJ, Gagliardi L (2008) The restricted active space followed by second-order perturbation theory method: Theory and application to the study of CuO2 and Cu2O2 systems. J Chem Phys 128(20):204109
Sauri V, Serrano-Andrés L, Shahi ARM, Gagliardi L, Vancoillie S, Pierloot K (2011) Multiconfigurational second-order perturbation theory restricted active space (RASPT2) method for electronic excited states: a benchmark study. J Chem Theo Comput 7:153–168
Aquilante F, De Vico L, Ferré N, Ghigo G, Malmqvist P-Å, Neogrády P, Pedersen TB, Pitoňák M, Reiher M, Roos BO, Serrano-Andrés L, Urban M, Veryazov V, Lindh R (2010) Molcas 7: The next generation. J Comput Chem 31(1):224–247
Veryazov V, Widmark P-O, Serrano-Andrés L, Lindh R, Roos BO (2004) 2molcas as a development platform for quantum chemistry software. Int J Quantum Chem 100(4):626–635
Karlström G, Lindh R, Malmqvist P-Å, Roos BO, Ryde U, Veryazov V, Widmark P-O, Cossi M, Schimmelpfennig B, Neogrady P, Seijo L (2003) Molcas: a program package for computational chemistry. Comput Mat Sci 28:222–239
Martin RL (2003) Natural transition orbitals. J Chem Phys 118:4775–4777
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, “Gaussian 16 Revision B01,” (2016). Gaussian Inc. Wallingford CT
Becker RS, Singh IS, Jackson EA (1963) Comprehensive spectroscopic investigation of polynuclear aromatic hydrocarbons. I. Absorption spectra and state assignments for the tetracyclic hydrocarbons and their alkyl-substituted derivatives. J Chem Phys 38:2144–2171
DMS UV Atlas of Organic Compounds. Verlag Chemie, Weinheim, and Butterworths London,(1971)
Salvi P, Foggi P, Castellucci E (1983) The two-photon excitation spectrum of pyrene. Chem Phys Lett 98:206–211
Jones CM, Asher SA (1988) Ultraviolet resonance Raman study of the pyrene \(S_{4}\), \(S_{3}\), and \(S_{2}\) excited electronic states. J Chem Phys 89:2649–2661
Thöny A, Rossi MJ (1997) Gas-phase UV spectroscopy of anthracene, xanthone, pyrene, 1-bromopyrene and 1,2,4-trichlorobenzene at elevated temperatures. J Photochem Photobiol, A 104:25–33
Baba M, Saitoh M, Kowaka Y, Taguma K, Yoshida K, Semba Y, Kasahara S, Yamanaka T, Ohshima Y, Hsu Y-C, Lin SH (2009) Vibrational and rotational structure and excited-state dynamics of pyrene. J Chem Phys 131:224318
Numata Y, Suzuki Y, Suzuka I (2012) Anomalous fluorescence from the second excited singlet state upon excitation to the S4 state of pyrene in a supersonic jet. J Photochem Photobiol, A 237:49–52
Rouillé G, Jäger C, Steglich M, Huisken F, Henning T, Theumer G, Bauer I, Knölker H-J (2008) IR, Raman, and UV/Vis Spectra of Corannulene for Use in Possible Interstellar Identification. ChemPhysChem 9:2085–2091
Feng M, Zhao J, Petek H (2008) Atomlike, Hollow-Core-Bound Molecular Orbitals of C\(_{\rm 60 }\). Science 320:359–362
Feng M, Zhao J, Huang T, Zhu X, Petek H (2011) The electronic properties of superatom states of hollow molecules. Acc Chem Res 44:360–368
Johansson JO, Henderson GG, Remacle F, Campbell EEB (2012) Angular-resolved Photoelectron Spectroscopy of Superatom Orbitals of Fullerenes. Phys Rev Lett 108:173401
Mignolet B, Johansson JO, Campbell EEB, Remacle F (2013) Probing Rapidly Ionizing Super Atom Molecular Orbitals in C \(_{\rm 60 }\) : A Computational and Femtosecond Photoelectron Spectroscopy Study. ChemPhysChem 14:3332–3340
Zhao J, Feng M, Yang J, Petek H (2009) The superatom states of fullerenes and their hybridization into the nearly free electron bands of fullerites. ACS Nano 3:853–864
Zoppi L, Martin-Samos L, Baldridge KK (2015) Buckybowl superatom states: a unique route for electron transport? Phys Chem Chem Phys 17(8):6114–6121
Turi L, Rossky PJ (2012) Theoretical studies of spectroscopy and dynamics of hydrated electrons. Chem Rev 112:5641–5674
Acknowledgements
The authors thank Joëlle Mascetti and Jennifer Noble for fruitful scientific discussions. This work has been funded by the French Agence Nationale de la Recherche (ANR) project PARCS ANR-13-BS08-005. For the purpose of open access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript (version arising from this submission). This work was also supported by the French research network EMIE (Edifices Moléculaires Isolés et Environnés, GDR 3533), and by the French National Program Physique et Chimie du Milieu Interstellaire (PCMI) of the CNRS/INSU with the INC/INP, co-funded by the CEA and the CNES.
Author information
Authors and Affiliations
Contributions
N.B, S.K and A.S. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work will be published under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Amor, N., Konate, S. & Simon, A. Electronic excited states of planar vs bowl-shaped polycyclic aromatic hydrocarbons in interaction with water clusters: a TD-DFT study. Theor Chem Acc 142, 74 (2023). https://doi.org/10.1007/s00214-023-03005-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03005-9