Abstract
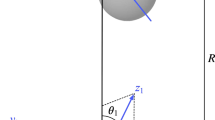
The potential energy surface (PES) of a \({\text{HeH}}_{3}^{ + }\) system for different orientations of monomers is calculated with the CCSD(T) method. The interaction energies obtained through aug-cc-pVDZ and aug-cc-pVTZ basis sets are extrapolated to a complete basis set limit using an extrapolated scheme. The study of the effects of different basis sets shows that the basis set used in this research is efficient in terms of accuracy and the time needed to perform calculations. To improve the quality of PES, fitting curves and the counterpoise correction method are used to determine the basis set superposition error for all the calculations. Finally, a three-dimensional plot of the intermolecular potential energy surface is drawn along with its contour plot of the potential intermolecular interaction. The second virial coefficient is also determined over a wide range of temperature for the He \({\text{H}}_{3}^{ + }\) system.







Similar content being viewed by others
References
Herbst E (1995) Chemistry in the interstellar medium. Annu Rev Phys Chem 46(1):27–54
Lembo L, Helm H, Huestis D (1989) Measurement of vibrational frequencies of the H3 molecule using two-step photoionization. J Chem Phys 90(10):5299–5308
Pelley J (2019) ACS Cent Sci 5:741–744. https://doi.org/10.1021/acscentsci.9b00441
Pauzat F, Ellinger Y, Pilmé J, Mousis O (2009) H3+ as a trap for noble gases-3: multiple trapping of neon, argon, and krypton in XnH3+(n = 1–3). J Chem Phys 130(17):174313. https://doi.org/10.1063/1.3126777
Pauzat F, Ellinger Y (2007) H3+ as a trap for noble gases-2: structure and energetics of X H3+ complexes from X = neon to xenon. J Chem Phys 127(1):014308. https://doi.org/10.1063/1.2746033
Pavanello M, Adamowicz L, Alijah A, Zobov NF, Mizus II, Polyansky OL, Tennyson J, Szidarovszky T, Császár AG, Berg M (2012) Precision measurements and computations of transition energies in rotationally cold triatomic hydrogen ions up to the midvisible spectral range. Phys Rev Lett 108(2):023002. https://doi.org/10.1103/PhysRevLett.108.023002
Drossart P, Maillard J-P, Caldwell J, Kim S, Watson J, Majewski W, Tennyson J, Miller S, Atreya S, Clarke J (1989) Detection of H3+ on Jupiter. Nature 340(6234):539–541
Thomson JJ (1913) Bakerian Lecture: Rays of positive electricity. Proc R Soc Lond Ser A Contain Pap Math Phys Char 89(607):1–20. https://doi.org/10.1098/rspa.1913.0057
Martin D, McDaniel E, Meeks M (1961) On the possible occurence of H3+ in interstellar space. Astrophys J 134:1012–1013
Watson WD (1973) The rate of formation of interstellar molecules by ion-molecule reactions. Astrophys J 183:L17
Herbst E, Klemperer W (1973) The formation and depletion of molecules in dense interstellar clouds. Astrophys J 185:505–534
Oka T (1980) Observation of the infrared spectrum of H3+. Phys Rev Lett 45(7):531
Geballe T, Jagod M-F, Oka T (1993) Detection of H3+ infrared emission lines in Saturn. Astrophys J 408:L109–L112
Trafton L, Geballe T, Miller S, Tennyson J, Ballester G (1993) Detection of H3+ from Uranus. Astrophys J 405:761–766
Geballe T, Oka T (1996) Detection of H3+ in interstellar space. Nature 384(6607):334–335
Oka T (2006) Interstellar H3+. Proc Natl Acad Sci 103(33):12235–12242
Oka T (2012) Chemistry, astronomy and physics of H3+. The Royal Society Publishing
Ray LC, Lorch C, O’Donoghue J, Yates J, Badman S, Smith C, Stallard TS (2019) Why is the H3+ hot spot above Jupiter’s Great Red Spot so hot? Phil Trans R Soc A 377(2154):20180407. https://doi.org/10.1098/rsta.2018.0407
Pavanello M, Adamowicz L, Alijah A, Zobov NF, Mizus II, Polyansky OL, Tennyson J, Szidarovszky T, Császár AG (2012) Calibration-quality adiabatic potential energy surfaces for H3+ and its isotopologues. J Chem Phys 136(18):184303
Ghosh S, Mukherjee S, Mukherjee B, Mandal S, Sharma R, Chaudhury P, Adhikari S (2017) Beyond Born-Oppenheimer theory for ab initio constructed diabatic potential energy surfaces of singlet H3+ to study reaction dynamics using coupled 3D time-dependent wave-packet approach. J Chem Phys 147(7):074105
Mukherjee S, Mukhopadhyay D, Adhikari S (2014) Conical intersections and diabatic potential energy surfaces for the three lowest electronic singlet states of H3+. J Chem Phys 141(20):204306
Mukherjee B, Mukherjee S, Adhikari S (2016) Ab-initio non-adiabatic couplings among three lowest singlet states of H3+: construction of multisheeted diabatic potential energy surfaces. J Phys: Conf Ser, 2016. vol 1. IOP Publishing, p 012050
Tennyson J, Polyansky OL, Zobov NF, Alijah A, Császár AG (2017) High-accuracy calculations of the rotation-vibration spectrum of. J Phys B: At Mol Opt Phys 50(23):232001
Aguado A, Roncero O, Sanz-Sanz C (2021) Three states global fittings with improved long range: singlet and triplet states of H3+. Phys Chem Chem Phys 23(13):7735–7747
Mizus II, Polyansky OL, McKemmish LK, Tennyson J, Alijah A, Zobov NF (2019) A global potential energy surface for H3+. Mol Phys 117(13):1663–1672. https://doi.org/10.1080/00268976.2018.1554195
Ravi S, Mukherjee S, Mukherjee B, Adhikari S, Sathyamurthy N, Baer M (2020) Non-adiabatic coupling as a frictional force in the formation of H3+: a model dynamical study. Eur Phys J D 74(12):1–13
Sanz-Sanz C, Aguado A, Roncero O (2021) Near-resonant effects in the quantum dynamics of the H+ H2+→ H2+ H+ charge transfer reaction and isotopic variants. J Chem Phys 154(10):104104
Benson MJ, McLaughlin DR (1972) Quantum mechanical investigation of the HeH3+ energy surface. J Chem Phys 56(3):1322–1331
Poshusta R, Agrawal V (1973) Stability of HeH3+. J Chem Phys 59(5):2477–2482
Asvany O, Brünken S, Kluge L, Schlemmer S (2014) COLTRAP: a 22-pole ion trapping machine for spectroscopy at 4 K. Appl Phys B 114(1):203–211
Savić I, Gerlich D, Asvany O, Jusko P, Schlemmer S (2015) Controlled synthesis and analysis of HeH3+ in a 3.7 K ion trap. Mol Phys 113(15–16):2320–2332
Coxon J (1971) The calculation of potential energy curves of diatomic molecules: application to halogen molecules. J Quant Spectrosc Radiat Transf 11(5):443–462
Gough D, Maitland G, Smith E (1972) The direct determination of intermolecular potential energy functions from gas viscosity measurements. Mol Phys 24(1):151–161
Balucani N, Beneventi L, Casavecchia P, Volpi G (1991) Dynamics of the reaction O (1D)+ HCl→ ClO+ H from crossed-beam experiments. Chem Phys Lett 180(1–2):34–40
McCourt FR, ter Horst MA, Jameson CJ (1995) N2–Kr interaction: a multiproperty analysis. J Chem Phys 102(14):5752–5760
Chapman WB, Schiffman A, Hutson JM, Nesbitt DJ (1996) Rotationally inelastic scattering in CH4+He, Ne, and Ar: state-to-state cross sections via direct infrared laser absorption in crossed supersonic jets. J Chem Phys 105(9):3497–3516
Trusler BJ, Zarari WW, MP (1997) Model intermolecular potentials and virial coefficients determined from the speed of sound. Mol Phys 90(5):695–704
Nyman G, Yu H-G (2000) Quantum theory of bimolecular chemical reactions. Rep Prog Phys 63(7):1001
Noorbala MR, Sabzyan H (2004) A MP2/6-31G* intermolecular potential energy surface for the F2–F2 system. THEOCHEM 678(1–3):67–76. https://doi.org/10.1016/j.theochem.2004.03.015
Maisuradze GG, Kawano A, Thompson DL, Wagner AF, Minkoff M (2004) Interpolating moving least-squares methods for fitting potential energy surfaces: analysis of an application to a six-dimensional system. J Chem Phys 121(21):10329–10338. https://doi.org/10.1063/1.1810477
Dunne LJ, Murrell JN, Jemmer P (2001) Analytical potential energy surface and quasi-classical dynamics for the reaction LiH (X, 1Σ+)+ H (2S)→ Li (2S)+ H2 (X, 1Σ+ g). Chem Phys Lett 336(1–2):1–6
Rocha CM, Varandas AJ (2021) A general code for fitting global potential energy surfaces via CHIPR method: Direct-Fit Diatomic and tetratomic molecules. Comput Phys Commun 258:107556
Bowman JM, Czako G, Fu B (2011) High-dimensional ab initio potential energy surfaces for reaction dynamics calculations. Phys Chem Chem Phys 13(18):8094–8111
Chen Q, Paul L, Conte R, Apurba N, Bowman JM (2021) Breaking the coupled cluster barrier for machine learned potentials of large molecules: the case of 15-atom acetylacetone. J Phys Chem Lett 12:4902–4909. https://doi.org/10.1021/acs.jpclett.1c01142
Collins MA (2016) Can systematic molecular fragmentation be applied to direct Ab initio molecular dynamics? J Phys Chem A 120(46):9281–9291
Salazar MR (2002) A completely general methodology for fitting three-dimensional ab initio potential energy surfaces. Chem Phys Lett 359(5–6):460–465
Okoshi M, Atsumi T, Nakai H (2015) Revisiting the extrapolation of correlation energies to complete basis set limit. J Comput Chem 36(14):1075–1082. https://doi.org/10.1002/jcc.23896
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev: Comput Mol Sci 2(1):73–78
Woon DE, Dunning Jr TH (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98 (2):1358–1371
Kendall RA, Dunning Jr TH, Harrison RJ (1992) Electron affinities of the first‐row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96(9):6796–6806
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90(2):1007–1023
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies: some procedures with reduced errors. Mol Phys 19(4):553–566
Chal/asiński G, Szczȩśniak MGM, Kendall RA (1994) Supermolecular approach to many‐body dispersion interactions in weak van der Waals complexes: He, Ne, and Ar trimers. J Chem Phys 101 (10):8860-8869
Cybulski SM, Chałasiński G (1992) Perturbation analysis of the supermolecule interaction energy and the basis set superposition error. Chem Phys Lett 197(6):591–598
Cybulski S, Chal/asiński G, Moszyński R (1990) On decomposition of second‐order Mo/ller–Plesset supermolecular interaction energy and basis set effects. J Chem Phys 92 (7):4357–4363
Chal, asiński G, Szczȩśniak M, Cybulski S (1990) Calculations of nonadditive effects by means of supermolecular Mo/ller–Plesset perturbation theory approach: Ar3 and Ar4. J Chem Phys 92(4):2481–2487
Coulson CA The electronic structure of. In: Mathematical Proceedings of the Cambridge Philosophical Society, 1935. vol 2. Cambridge University Press, pp 244–259
Adamowicz L, Pavanello M (2012) Progress in calculating the potential energy surface of H3+. Philos Trans R Soc A: Math Phys Eng Sci 370:5001–5013. https://doi.org/10.1098/rsta.2012.0101
Alipour M (2014) Theoretical determination of the differential polarizability and anisotropy of alkaline earth oxide nanoclusters (BeO) n [n= 2–9]: The basis set and electron correlation effects. Int J Quantum Chem 114(4):255–260
Huh SB, Lee JS (2003) Basis set and correlation dependent extrapolation of correlation energy. J Chem Phys 118(7):3035–3042
Helgaker T, Klopper W, Koch H, Noga J (1997) Basis-set convergence of correlated calculations on water. J Chem Phys 106(23):9639–9646
Tashakor S, Noorbala MR, Namazian M (2016) F2 dimer: Improved intermolecular potential energy surface using ab initio calculations. Int J Quantum Chem 116(20):1477–1485. https://doi.org/10.1002/qua.25216
Tashakor S, Noorbala MR, Namazian M (2017) Ab initio study of the intermolecular potential energy surface for the ground electronic state of the O2–CO system and prediction of second virial coefficients. Theor Chem Acc 136(10):1–10. https://doi.org/10.1007/s00214-017-2158-z
Tashakor S, Noorbala MR, Payvandy P, Mohammadi-Manesh H, Namazian M (2018) Introducing a novel method based on the imperialistic competitive algorithm to determine fluorine intermolecular potential from ab initio calculations and calculation of some properties via MD simulations. Mol Simul 44(3):243–253. https://doi.org/10.1080/08927022.2017.1366655
Feller D, Sordo JA (2000) Performance of CCSDT for diatomic dissociation energies. J Chem Phys 113(2):485–493
Wilson S (2003) Handbook of molecular physics and quantum chemistry, vol 2. Wiley
Acknowledgements
We gratefully acknowledge Yazd University Research Council for its financial support. Habib Janipour also thanks the school of graduate studies at Yazd University for the award of a Ph.D. scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janipour, H., Noorbala, M.R. & Namazian, M. Calculation of the intermolecular potential energy surfaces of \({\mathbf{H}\mathbf{e}\mathbf{H}}_{3}^{+}\) by means of ab initio methods. Theor Chem Acc 141, 45 (2022). https://doi.org/10.1007/s00214-022-02905-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02905-6