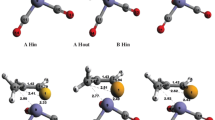
Abstract
In this paper, we will study the reactivity along with substituent changes in the OH insertion reaction in copper carbenoids. To this end, we have used M06-2X functional with cc-pVDZ for light atoms and LanL2DZ for copper. We have studied the IRC insertion profiles and analysed reactivity indexes such as electrophilicity (ω) and pKa calculations. We have found that with R1 substitutions phenyl group, R2 substitutions amide group lower the reaction barrier considerably. Concerning the substrate reactions, the most favoured substituent is NO2 in para position.













Similar content being viewed by others
References
Yudin AK (ed) (2010) Catalyzed carbon-heteroatom bond formation. Wiley-VCH Verlag GmbH & Co. KGaA, Wein heim. https://doi.org/10.1002/9783527633388.
Keipour H, Carreras V, Ollevier T (2017) Recent progress in the catalytic carbene insertion reactions into the silicon-hydrogen bond. Org Biomol Chem 15:5441–5456. https://doi.org/10.1039/C7OB00807D
Martín N, Dusselier M, De Vos DE, Cirujano FG (2019) Metal-organic framework derived metal oxide clusters in porous aluminosilicates: a catalyst design for the synthesis of bioactive aza-heterocycles. ACS Catal 9:44–48. https://doi.org/10.1021/acscatal.8b03908
Liu W, Cheng MJ, Nielsen RJ, Goddard WA, Groves JT (2017) Probing the C-O bond-formation step in metalloporphyrin-catalyzed C-H oxygenation reactions. ACS Catal 7:4182–4188. https://doi.org/10.1021/acscatal.7b00655
Lohr TL, Li Z, Marks TJ (2016) Thermodynamic strategies for C-O bond formation and cleavage via tandem catalysis. Acc Chem Res 49:824–834. https://doi.org/10.1021/acs.accounts.6b00069
Cheng XF, Li Y, Su YM, Yin F, Wang JY, Sheng J, Vora HU, Wang X-S, Yu J-Q (2013) Pd(II)-catalyzed enantioselective C–H activation/C-O bond formation: synthesis of chiral benzofuranones. J Am Chem Soc 135:1236–1239. https://doi.org/10.1021/ja311259x
Manna K, Kruse ML, Sadow AD (2011) Concerted C-N/C–H bond formation in highly enantioselective yttrium(III)-catalyzed hydroamination. ACS Catal 1:1637–1642. https://doi.org/10.1021/cs200511z
Kuwabe SI, Torraca KE, Buchwald SL (2001) Palladium-catalyzed intramolecular C-O bond formation. J Am Chem Soc 123:12202–12206. https://doi.org/10.1021/ja012046d
Besora M, Braga AAC, Sameera WMC, Urbano J, Fructos MR, Perez PJ, Maseras F (2015) A computational view on the reactions of hydrocarbons with coinage metal complexes. J Organometall Chem 784:2–12. https://doi.org/10.1016/j.jorganchem.2014.10.009
Xia Y, Qiu D, Wang J (2017) Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem Rev 117:13810–13889. https://doi.org/10.1021/acs.chemrev.7b00382
Ren YY, Zhu SF, Zhou QL (2018) Chiral proton-transfer shuttle catalysts for carbene insertion reactions. Org Biomol Chem 16:3087–3094. https://doi.org/10.1039/C8OB00473K
Yuan H, Nuligonda T, Gao H, Tung C-H, Xu Z (2018) Copper-catalyzed carbene insertion into the sulfur-sulfur bond of benzenesulfonothioate. Org Chem Front 5:1371–1374. https://doi.org/10.1039/C7QO01131H
Hyde S, Veliks J, Liégault B, Grassi D, Taillefer M, Gouverneur V (2016) Copper-catalyzed insertion into heteroatom-hydrogen bonds with trifluorodiazoalkanes. Angew Chem 128:3849–3853. https://doi.org/10.1002/ange.201511954
Ramakrishna K, Sivasankar C (2017) Iridium catalyzed acceptor/acceptor carbene insertion into N-H bonds in water. Org Biomol Chem 15:2392–2396. https://doi.org/10.1039/C7OB00177K
Fraile JM, L´opez-Ram-de-Viu P, Mayoral JA, Rold´an M, Santaf´e-Valero J (2011) Enantioselective C–H carbene insertions with homogeneous and immobilized copper complexes. Org Biomol Chem 9:6075. https://doi.org/10.1039/c1ob05499f
Davies HML, Beckwith REJ (2003) Catalytic enantioselective C–H activation by means of metal-carbenoid-induced C–H insertion. Chem Rev 103:2861–2903. https://doi.org/10.1021/cr0200217
Wang S, Xu S, Yang C, Sun H, Wang J (2019) Formal carbene C–H bond insertion in the Cu(I)-catalyzed reaction of bis(trimethylsilyl)diazomethane with benzoxazoles and oxazoles. Org Lett 21:1809–1812. https://doi.org/10.1021/acs.orglett.9b00391
Xia Y, Liu Z, Liu Z, Ge R, Ye F, Hossain M, Zhang Y, Wang J (2014) Formal carbene insertion into C-C bond: Rh(I)-catalyzed reaction of benzocyclobutenols with diazoesters. J Am Chem Soc 136:3013–3015. https://doi.org/10.1021/ja500118w
Zhu SF, Zhou QL (2012) Transition-metal-catalyzed enantioselective heteroatom-hydrogen bond insertion reactions. Acc Chem Res 45:1365–1377. https://doi.org/10.1021/ar300051u
Gillingham D, Fei N (2013) Catalytic X-H insertion reactions based on car- benoids. Chem Soc Rev 42:4918–4931. https://doi.org/10.1039/c3cs35496b
Xia Y, Zhang Y, Wang J (2013) Catalytic cascade reactions involving metal carbene migratory insertion. ACS Catal 3:2586–2598. https://doi.org/10.1021/cs4006666
Keipour H, Jalba A, Tanbouza N, Carreras V, Ollevier T (2019) α-Thiocarbonyl synthesis via the FeII-catalyzed insertion reaction of α-diazocarbonyls into S-H bonds. Org Biomol Chem 17:3098–3102. https://doi.org/10.1039/c9ob00261h
Durán R, Herrera B (2020) Theoretical study of the mechanism of catalytic enantioselective n-h and o-h insertion reactions. J Phys Chem 124:2–11. https://doi.org/10.1021/acs.jpca.9b07274
Parr RG, Szentpály L, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Chamorro E, Chattaraj PK, Fuentealba P (2003) Variation of the electrophilicity index along the reaction path. J Phys Chem A 107:7068–7072. https://doi.org/10.1021/jp035435y
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107:46–74. https://doi.org/10.1021/cr078014b
Pérez P, Aizman A, Contreras R (2002) Comparison between experimental and theoretical scales of electrophilicity based on reactivity indexes. J Phys Chem A 106:3964–3966. https://doi.org/10.1021/jp014664m
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106(14):4049–4050
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Mardirossian N, Head-Gordon M (2016) How accurate are the Minnesota density functionals for noncovalent interactions, isomerization energies, thermochemistry, and barrier heights involving molecules composed of main-group elements? J Chem Theory Comput 12:4303–4325. https://doi.org/10.1021/acs.jctc.6b00637
Lin YS, Li GD, Mao SP, Chai JD (2013) Long-range corrected hybrid density functionals with improved dispersion corrections. J Chem Theory Comput 9:263–272. https://doi.org/10.1021/ct300715s
Riplinger C, Neese F (2013) An efficient and near linear scaling pair natural orbital based local coupled cluster method. J Chem Phys 138:034106. https://doi.org/10.1063/1.4773581
Lian P, Johnston RC, Parks JM, Smith JC (2018) Quantum chemical calculation of pKas of environmentally relevant functional groups: carboxylic acids, amines, and thiols in aqueous solution. J Phys Chem A 122:4366–4374. https://doi.org/10.1021/acs.jpca.8b01751
Fukui K (1981) The path of chemical reactions—the IRC approach. Acc Chem Res 14:363–368. https://doi.org/10.1021/ar00072a001
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H (2016) Gaussian Inc 16, revision B.01. Gaussian Inc., Wallingford
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci 2:73–78. https://doi.org/10.1002/wcms.81
Zhurko G, Zhurko D (2017) ChemCraft, Version 1.8. http://www.chemcraftprog.com
Legault C (2009) CYLview, Version 1.0b. http://www.cylview.org
Boche G, Lohrenz JCW (2001) The electrophilic nature of carbenoids, nitrenoids, and oxenoids. Chem Rev 101:697–756. https://doi.org/10.1021/cr940260x
Davies HML, Antoulinakis EG (2001) Recent progress in asymmetric intermolecular C-H activation by rhodium carbenoid intermediates. J Organometall Chem 617–618:47–55. https://doi.org/10.1016/S0022-328X(00)00599-4
Funding
This article was funded by ANID FONDECYT REGULAR No. 1170837.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not declare conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Durán, R., Herrera, B. Theoretical study of the substituent effect on the O–H insertion reaction of copper carbenoids. Theor Chem Acc 141, 15 (2022). https://doi.org/10.1007/s00214-022-02876-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02876-8