Abstract
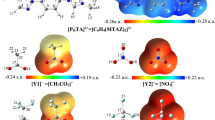
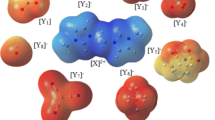
Some of the quantum chemical computable characteristics of the new designed di-cationic ILs ([X][Y1–6]2) (X = [p − C6H4(CH2MIM)2]2+ and Y1–6 = CH3CO2–, CF3CO2–, BF4–, ClO4–, CF3SO3– and PF6–) based on para-xylyl linked bis-(3-methyl-1-imidazolium) cation and various anions were investigated using density functional theory (DFT) at M062X/aug-ccpvdz level of theory. The geometrical characteristics, electrostatic potential maps, dispersion including interaction energies, natural charge and charge transfer (CT), topological specifications, reduced gradient density (RGD) plots, cathodic (VCL) and anodic (VAL) limits of potentials as well as electrochemical windows (ECW) were explored and evaluated. The obtained consequences for dispersion corrected interaction energies which are the consequences of the intermolecular hydrogen bonding and electrostatic interactions (− 212.06 to − 250.22 kcal mol−1) showed the following order [X][Y1]2 > [X][Y2]2 > [X][Y3]2 > [X][Y4]2 > [X][Y5]2 > [X][Y6]2. The electrostatic essence for the C–H⋅⋅⋅O and C–H⋅⋅⋅F hydrogen bonds in [X][Y1–6]2 D-ILs is verified by the consequences of the electron density analysis calculations. The following order for ECWs of the designed di-cationic ILs (D-ILs) was obtained: [X][CH3CO2]2 < [X][CF3SO3]2 < [X][CF3CO2]2 ≅ [X][BF4]2 ≅ [X][ClO4]2 ≅ [X][PF6]2. For D-ILs composed of CF3CO2–, BF4–, ClO4– and PF6– anions, it is predicted that the anodic stability limit in aqueous environment is controlled by the di-cation constructing part of the D-ILs.










Similar content being viewed by others
References
Priyanka VP, Gardas RL (2020) Mono and di-cationic ionic liquids based aqueous biphasic systems for the extraction of diclofenac sodium. Sep Purif Technol 234:116048
Roohi H, Fallah Ghasemi Gildeh S, Mehrdad M, Ghauri K (2020) Exploring the physicochemical properties of para-xylyl linked DBU-based di-cationic ionic liquids consist of various anions: a GD3–M06–2X study. J Mole Liquid 310:113060
Roohi H, Fallah Ghasemi Gildeh S, Ghauri K, Fathei P (2020) Physicochemical properties of the imidazolium-based di-cationic ionic liquids (DILs) composed of ethylene π-spacer by changing the anions: a quantum chemical approach. Ionics 26:1963
Vélez JF, Vázquez-Santos MB, Amarilla JM, Herradón B, Mann E, del Río C, Morales E (2019) Geminalpyrrolidinium and piperidinium di-cationic ionic liquid electrolytes Synthesis characterization and cell performance in LiMn2O4 rechargeable lithium cells. J Power Sourc 439:227098
Alavi SM, Yeganegi S (2018) DFT study of structures and hydrogen bonds of imidazolium-based halogen-free boron containing di-cationic ionic liquids. J Mol Liq 256:330
Haddad B, Paolone A, Villemin D, Lohier J-F, Drai M, Bresson S, Abbas O, Belarbi E (2018) Para-Xylyl bis-1-methylimidazolium bis(trifluoromethanesulfonyl)imide: synthesis, crystal structure, thermal stability, Vibrational Studies. J Mol Liq 260:391
Masri AN, Mutalib Mi A, Leveque J-M (2016) A review on di-cationic ionic liquids: classification and application. Ind Eng Manag 5:197–203
Rodrigues M, Russo L, Aguiló E, Rodríguez L, Ott I, Pérez-García L (2016) Au (I) N-heterocyclic carbenes from bis-imidazolium amphiphiles: synthesis, cytotoxicity and incorporation onto gold nanoparticles. RSC Adv 6:2202
Azizi A, Shirdel NF (2016) Task specific di-cationic acidic ionic liquids catalyzed efficient and rapid synthesis of benzoxanthenones derivatives. J Mol Liq 222:783
Mahrova M, Pagano F, Pejakovic V, Valea A, Kalin M, Igartua A, Tojoet E (2015) Pyridinium based di-cationic ionic liquids as base lubricants or lubricant additives. Tribol Int 82:245
Farmanzadeh D, Soltanabadi A, Yeganegi S (2013) DFT study of the geometrical and electronic structures of geminal di-cationic ionic liquids 1,3-bis[3-methylimidazolium-1-yl] hexane halides. J Chin Chem Soc 60:551
Shirota H, Mandai T, Fukazawa H, Kato T (2011) Comparison between di-cationic and mono-cationic ionic liquids: liquid density, thermal properties, surface tension, and shear viscosity. J Chem Eng Data 56:2453
Liang JH, Ren XQ, Wang JT, Jinag M, Li ZJ (2010) Preparation of biodiesel by transesterification from cottonseed oil using the basic di-cation ionic liquids as catalysts. J Fuel Chem Technol 38:275
Payagala T, Huang J, Breitbach ZS, Sharma PS, Armstrong DW (2007) Unsymmetrical di-cationic ionic liquids: manipulation of physicochemical properties using specific structural architectures. Chem Mater 19:5848
Anderson JL, Ding R, Ellern A, Armstrong DW (2005) Structure and properties of high stability geminal di-cationic ionic liquids. J Am Chem Soc 127:593
Moumene T, Belarbi EH, Haddad B, Villemin D, Abbas O, Khelifa B, Bresson S (2014) Vibrational spectroscopic study of ionic liquids: comparison between mono-cationic and di-cationic imidazolium ionic liquids. J Mol Struc 1065–1066:86
Talebi M, Patil RA, Armstrong DW (2018) Physicochemical properties of branched-chain di-cationic ionic liquids. J Mol Liq 256:247
Mei X, Yue Z, Ma Q, Dunya H, Mandal BK (2018) Synthesis and electrochemical properties of new di-cationic ionic liquids. J Mol Liq 272:1001
Boumediene M, Haddad B, Paolone A, Drai M, Villemin D, Rahmouni M, Bresson S, Abbas O (2019) Synthesis, thermal stability, vibrational spectra and conformational studies of novel di-cationic meta-xylyl linked bis-1-methylimidazolium ionic liquids. J Mol Struct 1186:68
Zhang H, Li M, Yang B (2018) Design, Synthesis, and analysis of thermo-physical properties for imidazolium-based geminal di-cationic ionic liquids. J Phys Chem C 122:2467
Patil RA, Talebi M, Xu C, Bhawal SS, Armstrong DW (2016) Synthesis of thermally stable geminal di-cationic ionic liquids and related ionic compounds: an examination of physicochemical properties by structural modification. Chem Mater 28:4315
Zekri N, Fareghi-Alamdari R, Khodarahmi Z (2016) Functionalized di-cationic ionic liquids: green and efficient alternatives for catalysts in phthalate plasticizers preparation. J Chem Sci 128:1277
Chang JC, Ho WY, Sun IW, Chou YK, Hsieh HH (2011) Synthesis and properties of new tetrachlorocobaltate (II) and tetrachloromanganate (II) anion salts with di-cationic counter-ions. Polyhedron 30:497
Chang JC, Ho WY, Sun IW, Tung YL, Tsui MC (2010) Synthesis and characterization of di-cationic ionic liquids that contain both hydrophilic and hydrophobic anions. Tetrahedron Lett 66:6150
Chang JC, Ho WY, Sun IW, Chou YK, Hsieh HH (2010) Synthesis and properties of new (μ-oxo) bis [trichloroferrate (III)] di-anion salts incorporated with di-cationic moiety. Polyhedron 29:2976
Zeng Z, Phillips BS, Xiao J-C, Shreeve JM (2008) Polyfluoroalkyl, polyethylene glycol, 1,4-bismethylenebenzene, or 1,4-bismethylene-2,3,5,6-tetrafluorobenzene bridged functionalized di-cationic ionic liquids: synthesis and properties as high temperature lubricants. Chem Mater 20:2719
Zhang ZX, Zhou HY, Yang L, Tachibana K, Kamijima K (2008) Asymmetrical di-cationic ionic liquids based on both imidazolium and aliphatic ammonium as potential electrolyte additives applied to lithium secondary batteries. Electrochim Acta 53:4833
Ding YS, Zha M, Zhang J, Wang SS (2007) Synthesis, characterization and properties of geminal imidazolium ionic liquids. Colloids Surf A Physicochem Eng Asp 298:201
Zhang Z, Yang L, Luo S, Tian M, Tachibana K (2007) Ionic liquids based on aliphatic tetra alkyl ammonium di-cations and TFSI anion as potential electrolytes. J Power Sourc 167:217
Talaei R, Khalili B, Mokhtary M (2020) Modulation of opto-electronic properties of the functionalized hexagonal boron nitride nano-sheets with tunable aryl alkyl ionic liquids (TAAILs): defect based analysis. J Mole Liq 304:112696
Khalili B, Rasoulian M, Ghauri K (2020) First time investigation of the substitution effect at anion part of the ILs on their physicochemical properties using [DMT][4-XPhSO3],(X = NH2, OH, H, F, Br, CHO, CF3, CN and NO2) as a model ILs: a systematic DFT study. J Mol Struct 1201:127171
Khalili B, Rimaz M (2017) An investigation on the physicochemical properties of the nanostructured [(4-X) PMAT][N(CN)2] ion pairs as energetic and tunable aryl alkyl amino tetrazolium based ionic liquids. J Mol Struct 1137:530
Khalili B, Rimaz M (2017) Does interaction between an amino acid anion and methyl imidazolium cation lead to a nanostructured ion pairs of [Mim][AA] as an ionic liquid? J Mol Liq 229:267
Roohi H, Khyrkhah S (2013) Ion-pairs formed in [Mim+][N(CN)2−] ionic liquid: structures, binding energies NMR SSCCs, volumetric, thermodynamic and topological properties. J Mole Liq 177:119
Sun H, Zhang D, Liu C, Zhang C (2009) Geometrical and electronic structures of the di-cation and ion pair in the germinal di-cationic ionic liquid 1,3-bis[3-methylimidazolium-yl] propane bromide. J Mol Struct (Thoechem) 900:37
Roohi H, Ghauri K (2016) Influence of various anions and cations on electrochemical and physicochemical properties of the nanostructured tunable aryl alkyl ionic liquids (TAAILs): a DFT M06–2X study. Thermochim Acta 639:20
Roohi H, Salehi R (2011) Molecular interactions in methyl imidazolium tetra fluoroborate ionic liquid ([Mim+][BF4−]): structures, binding energies, topological properties and NMR one-and two bonds spin–spin coupling constants. J Mol Liq 161(2):63
Zhao Y, Truhlar DG, Theor (2008) The M06 suite of density functional for main group, thermochemistry thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functional and systematic testing of four M06-class functional and 12 other functional. Chem Acc 120:215
Zhao Y, Truhlar DG (2006) Comparative DFT study of van der Waals complexes: rare-gas dimers, alkaline-earth dimers, zinc dimer, and zinc-rare-gas dimers. J Phys Chem 110:5121
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, revision A.02, Gaussian 09 Citation, Gaussian, Inc, Wallingford CT
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1995) Department 995 of chemistry. University of California-Irvine, Irvine, CA
Bader R (1990) Atoms in molecules: a quantum theory, vol 997. Oxford University Press, New York
Biegler-König F, Schönbohm J, Bayles D (2001) J Comput Chem 998(22):545
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625
Lu T, Chen F (2012) Multiwfn: a multifunctional wave function analyzer. J Comput Chem 33:580
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Haddad B, Paolone A, Drai M, Boumediene M, Villemin D, Belarbi E, Rahmouni M, Bresson S, Abbas O (2019) Para-xylyl linked bis-imidazolium ionic liquids: a study of the conformers of the cation and of the anion-cation hydrogen bonding. J Mol Struct 1175:175
Schulz T, Ahrens S, Meyer D, Allolio C, Peritz A, Strassner T (2011) Electronic effects of para-substitution on the melting points of TAAILs. Chem Asian J 6:863
Pitawala J, Matic A, Martinelli A, Jacobsson P, Koch V, Croce F (2009) Thermal properties and ionic conductivity of imidazoliumbis(trifluoromethanesulfonyl)imide di-cationic ionic liquids. J Phys Chem B 113:10607
Izgorodina EI, Bernard UL, MacFarlane DR (2009) Ion-pair binding energies of ionic liquids: can DFT compete with ab initio-based methods? J Phys Chem A 113:7064
Ghatee MH, Moosavi F, Zolghadr AR, Jahromi R (2010) Critical-point temperature of ionic liquids from surface tension at liquid−vapor equilibrium and the correlation with the interaction energy. Ind Eng Chem Res 49:12696
Reed AE, Curtiss LA, Weinhold F (1998) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899
Koch VR, Dominey LA, Nanjundiah C (1996) The intrinsic anodic stability of several anions comprising solvent-free ionic liquids. J Electrochem Soc 143:798
Kroon MC, Buijs W, Peters CJ, Witkamp GJ (2006) Decomposition of ionic liquids in electrochemical processing. J Green Chem 8:241
Buijs WGJ, Witkamp MC (2012) Kroon, Correlation between quantum chemically calculated lumo energies and the electrochemical window of ionic liquids with reduction-resistant anions. Int J Electrochem 589050:1
Wu P, Chaudret R, Hu X, Yang W (2013) Noncovalent interaction analysis in fluctuating environments. J Chem Theory Comput 9:2226
Ong SP, Andreussi O, Wu Y, Marzari N, Ceder G (2011) Electrochemical windows of room-temperature ionic liquids from molecular dynamics and density functional theory calculations. Chem Mater 23:2979
Ue M, Murakami A, Nakamurab S (2002) A convenient method to estimate ion size for electrolyte materials design. J Electrochem Soc 149:1572
Kazemiabnavi S, Zhang Z, Thornton K, Banerjee S (2016) Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries. J Phys Chem B 120:5691
Fallah Ghasemi Gildeh S, Roohi H, Mehrdad M, Rad-Moghadam K, Ghauri K (2020) Experimental and theoretical probing of the physicochemical properties of ionic liquids composed of [Bn-DBU] cation and various anions. J Mol Struct 1202:127226
Daily LA, Miller KM (2013) Correlating structure with thermal properties for a series of 1-alkyl-4-methyl-1,2,4-triazolium ionic liquids. J Org Chem 78:4196
Acknowledgements
Support of this work from the Research Council of the University of Guilan is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalili, B., Mamaghani, M. & Bazdid-Vahdati, N. Structural design and physicochemical specifications exploring of the new di-cationic ionic liquids (D-ILs) composed of para-xylyl linked N-Methylimidazolium cation and various anions: a full M06–2X computational study. Theor Chem Acc 141, 3 (2022). https://doi.org/10.1007/s00214-021-02862-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02862-6