Abstract
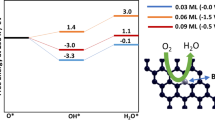
The adsorption properties of the intermediates of oxygen reduction reaction on bismuthene and graphene/bismuthene heterojunctions have been studied, and the oxygen reduction reaction processes have been simulated. The five intermediates, O, OH, O2, OOH and H2O, all tend to adsorb on the vacancy sites of the bismuthene, with adsorption energies of − 4.42 eV, − 3.48 eV, − 2.08 eV, − 1.94 eV and − 0.25 Ev, respectively. The adsorption energies of intermediates adsorbed on the bismuthene side in the heterojunction interspace decrease to − 4.31 eV, − 3.15 eV, − 1.60 eV, − 0.93 eV and 0.64 eV, respectively, resulting from space confinement effect. For oxygen reduction reaction, both bismuthene and graphene/bismuthene heterojunction show certain catalytic activity. The free energy changes of each step are negative, showing a spontaneous trend of reaction on the bismuthene. While both two-electron and four-electron processes exist on the bismuthene, there are solely four-electron processes that exist on the heterojunction, avoiding the production of H2O2 which is detrimental to the catalyst and the proton exchange membrane.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Liu X, Li L, Meng C, Han Y (2012) Palladium nanoparticles/defective graphene composites as oxygen reduction electrocatalysts: a first-principles study. J Phys Chem C 116:2710–2719
Neergat M, Shukla AK, Gandhi KS (2001) Platinum-based alloys as oxygen–reduction catalysts for solid–polymer–electrolyte direct methanol fuel cells. J Appl Electrochem 31:373–378
Yu X, Ye S (2007) Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC - Part II: degradation mechanism and durability enhancement of carbon supported platinum catalyst. J Power Sources 172:145–154
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Qu L, Liu Y, Baek J, Dai L (2010) Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4:1321–1326
Li R, Wei Z, Gou X, Xu W (2013) Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. RSC Adv 3:9978–9984
Sheng ZH, Gao HL, Bao WJ, Wang FB, Xia XH (2011) Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J Mater Chem 22:390–395
Yang Z, Yao Z, Li G, Fang G, Nie H, Liu Z, Zhou X, Chen X, Huang S (2012) Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 6:205–211
Choi CH, Chung MW, Kwon HC, Park SH, Woo SI (2013) B, N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J Mater Chem A 1:3694–3699
Sun JP, Zhou KL, Liang XD (2016) Density functional study on the adsorption characteristics of O, O-2, OH, and OOH of B-, P-doped, and B P codoped graphenes. Acta Phys Sin 65:018201
Reich ES (2014) Phosphorene excites materials scientists. Nature 506:19
Pang J, Bachmatiuk A, Yin Y, Trzebicka B, Zhao L, Fu L, Mendes RG, Gemming T, Liu Z, Rummeli MH (2018) Applications of Phosphorene and Black Phosphorus in Energy Conversion and Storage Devices. Adv Energy Mater 8(8):1702093
Rahman MZ, Kwong CW, Davey K, Qiao SZ (2016) 2D Phosphorene as a water splitting photocatalyst: fundamentals to applications. Energy Environ Sci 9:709–728
Haldar S, Mukherjee S, Ahmed F, Singh CV (2017) A first principles study of hydrogen storage in lithium decorated defective phosphorene. Int J Hydrogen Energy 42:23018–23027
Lu Z, Pang Y, Li S, Wang Y, Yang Z, Ma D, Wu R (2019) Phosphorene: a promising metal free cathode material for proton exchange membrane fuel cell. Appl Surf Sci 479:590–594
Favron A, Gaufres E, Fossard F, Phaneuf-L’Heureux A, Tang NY, Levesque PL, Loiseau A, Leonelli R, Francoeur S, Martel R (2015) Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat Mater 14:826
Hu Y, Sun J, Wei H, Ai M, Li Z (2020) Adsorption characteristics and oxygen reduction reactions on pristine and Pt-, Co-decorated antimonenes: a DFT-D study. New J Chem 44:1138–1146
Sun H, Wang M, Zhu F, Wang G, Ma H, Xu Z, Liao Q, Lu Y, Gao C, Li Y, Liu C, Qian D, Guan D, Jia J (2017) Coexistence of topological edge state and superconductivity in bismuth ultrathin film. Nano Lett 17:3035–3039
Hussain N, Liang TX, Zhang QY, Anwar T, Huang Y, Lang JL, Huang K, Wu H (2017) Ultrathin bi nanosheets with superior photoluminescence. Small 13:1701349
Lu L, Liang Z, Wu L, Chen Y, Song Y, Dhanabalan SC, Ponraj JS, Dong B, Xiang Y, Xing F, Fan D, Zhang H (2018) Few-layer bismuthene: sonochemical exfoliation, nonlinear optics and applications for ultrafast photonics with enhanced stability. Laser Photonics Rev 12(1):170221
Reis F, Li G, Dudy L, Bauernfeind M, Glass S, Hanke W, Thomale R, Schafer J, Claessen R (2017) Bismuthene on a SiC substrate: a candidate for a high-temperature quantum spin Hall material. Science 357(6348):287–290
Zhang S, Xie M, Li F, Yan Z, Li Y, Kan E, Liu W, Chen Z, Zeng H (2016) Semiconducting group 15 Monolayers: a broad range of band gaps and high carrier mobilities. Angew Chem Int Ed 55:1666–1669
Xiao S, Wei D, Jin X (2012) Bi(111) Thin Film with Insulating Interior but Metallic Surfaces. Phys Rev Lett 109(16):166805
Atkins PW (1998) Physical Chemistry, 6th edn. OxfordUniversityPress, Oxford
Norskov JK, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin JR, Bligaard T, Jonsson H (2004) Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B 108:17886–17892
Zhang S, Guo S, Chen Z, Wang Y, Gao H, Gomez-Herrero J, Ares P, Zamora F, Zhu Z, Zeng H (2018) Recent progress in 2D group-VA semiconductors: from theory to experiment. Chem Soc Rev 47:982–1021
Kecik D, Ozcelik VO, Durgun E, Ciraci S (2019) Structure dependent optoelectronic properties of monolayer antimonene, bismuthene and their binary compound. Phys Chem Chem Phys 21:7907–7917
Alvim RD, Borges I, Leitao AA (2018) Proton migration on perfect, vacant, and doped MgO (001) Surfaces: role of dissociation residual groups. J Phys Chem C 122:21841–21853
Giovannetti G, Khomyakov PA, Brocks G, Kelly PJ, van den Brink J (2007) Substrate-induced band gap in graphene on hexagonal boron nitride: Ab initio density functional calculations. Phys Rev B Condens Matter Mater Phys 76:073103
Funding
Supported by the “National Natural Science Foundation of China” (Grant No. 61372050).
Author information
Authors and Affiliations
Contributions
ZL contributed to conceptualization, methodology, software, data curation, writing—original draft preparation. JS was involved in data curation, writing—original draft preparation, supervision, writing—reviewing and editing. HL contributed to software, visualization, investigation. MA was involved in software, validation. JH contributed to data curation, resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Sun, J., Liang, H. et al. Adsorption properties of the intermediates of oxygen reduction reaction on bismuthene and graphene/bismuthene heterojunction based on DFT study. Theor Chem Acc 140, 103 (2021). https://doi.org/10.1007/s00214-021-02814-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02814-0