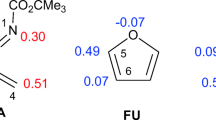
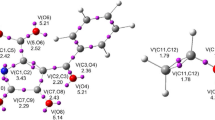
Abstract
The Diels–Alder cycloaddition of 3-bromo-1-phenylprop-2-ynone with furan and 2-methylfuran has been studied using the molecular electron density theory through DFT calculation at the B3LYB/6-311G (d,p) level of theory. The mechanism of regioselectivity for these reactions was investigated by the potential energy surface determination for the cycloaddition process. The regiostereoselectivity was studied by exploiting the local and global reactivity descriptors defined by Fukui and Parr functions; the results showed that the diene acts as a nucleophile, by two possible approaches, namely furan and 2-methylfuran, and the dienophile behaves as an electrophile. The prediction of the possible stereo-/regioisomers for this reaction is revealed by different preferable pathways of attack. The reaction mechanism has been followed by optimizing the transition state structure using the intrinsic coordinate IRC computations and the estimated activation energies. The analysis geometries of the transition states prove that the cycloaddition is established in one step according to an asynchronous mechanism for the various rotamers.




Similar content being viewed by others
References
Diels O, Alder K (1928) Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann Chem 460:98–122
Zhang C, Wang Z, Song J (2019) Bonding and Diels-Alder reactions of substituted 2-borabicyclo(1.1.0) but-1(3)-enes: a theoretical study. Theor Chem Acc 138:106–114
Fleming I (2015) Pericyclic reactions, 2nd edn. Oxford University Press, New York
Smith LJ, Taimoory SM, Tam RY (2018) Diels-Alder click-cross-linked hydrogels with increased reactivity enable 3D cell encapsulation. Biomacromol 19:926–935
Baalmann M, Best M, Wombacher R (2018) Site-specific protein labeling utilizing lipoic acid ligase (LplA) and bioorthogonal inverse electron demand Diels-Alder reaction. In: Lemke EA (ed) Noncanonical amino acids. Springer, New York, pp 365–387
Hirao H, Ohwada T (2005) Theoretical revisit of regioselectivities of Diels−Alder reactions: orbital-based reevaluation of multicentered reactivity in terms of reactive hybrid orbitals. J Phys Chem A 109:816–824
Beniwal V, Kumar A (2017) Thermodynamic and molecular origin of interfacial rate enhancements and endo-selectivities of a Diels-Alder reaction. Phys Chem Chem Phys 19:4297–4306
Smith NM, Swaminathan Iyer K, Corry B (2014) The confined space inside carbon nanotubes can dictate the stereo- and regioselectivity of Diels-Alder reactions. Phys Chem Chem Phys 16:6986
Krokidis X, Silvi B, Alikhani ME (1998) Topological characterization of the isomerization mechanisms in XNO (X=H, Cl). Chem Phys Lett 292:35–45
Fernández I, Bickelhaupt FM (2014) Origin of the “endo rule” in Diels-Alder reactions. J Comput Chem 35:371–376
Lam Y, Cheong PH-Y, Blasco Mata JM et al (2009) Diels−Alder exo selectivity in terminal-substituted dienes and dienophiles: experimental discoveries and computational explanations. J Am Chem Soc 131:1947–1957
Petrov V, Dooley RJ, Marchione AA, Diaz EL, Clem BS (2019) Simple protocol for preparation of Diels-Alder adducts of perfluorinated thioketones. J Fluorine Chem 225:1–10
Bakalova SM, Kaneti J (2008) Origins of stereoselectivity in the Diels−Alder addition of chiral hydroxyalkyl vinyl ketones to cyclopentadiene: a quantitative computational study †. J Phys Chem A 112:13006–13016
Bansal RK, Gupta N, Kumawat SK (2006) Origin of the stereo- and regioselectivities in the Diels-Alder reactions of azaphospholes: a DFT investigation. Tetrahedron 62:1548–1556
Ho G-M, Huang C-J, Li EY-T et al (2016) Unconventional exo selectivity in thermal normal-electron-demand Diels-Alder reactions. Sci Rep 6:35147–35156
Wolters LP, Bickelhaupt FM (2015) The activation strain model and molecular orbital theory: activation strain model and molecular orbital theory. WIREs Comput Mol Sci 5:324–343
Xu L, Doubleday CE, Houk KN (2010) Dynamics of 1,3-dipolar cycloadditions: energy partitioning of reactants and quantitation of synchronicity. J Am Chem Soc 132:3029–3037
Yang Y-F, Liang Y, Liu F, Houk KN (2016) Diels-Alder reactivities of benzene, pyridine, and Di-, Tri-, and tetrazines: the roles of geometrical distortions and orbital interactions. J Am Chem Soc 138:1660–1667
von Hopffgarten M, Frenking G (2012) Energy decomposition analysis. WIREs Comput Mol Sci 2:43–62
Petrov V, Marchione AA, Dooley R (2018) Diels-Alder reactions of “in situ” generated perfluorinated thioketones. Chem Commun 54:9298–9300
Fernández I (2014) Combined activation strain model and energy decomposition analysis methods: a new way to understand pericyclic reactions. Phys Chem Chem Phys 16:7662–7671
Chow R, Mok DKW, Lee EPF, Dyke JM (2016) A theoretical study of the atmospherically important radical–radical reaction BrO + HO2; the product channel O2(a1Δg) + HOBr is formed with the highest rate†. Phys Chem Chem Phys 18:30554–30569
Pal J, Subramanian R (2019) Theoretical studies of hydrogen abstraction from H 2X and CH 3XH (X = O, S) by trichloromethyl radicals. Phys Chem Chem Phys 21:6525–6534
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. a theoretical study †. J Org Chem 73:4615–4624
Tomilin DN, Sobenina LN, Gotsko MD et al (2018) Reaction of 1-(het)aryl-3-bromoprop-2-ynones with furans in solid metal oxides or salts: cross-coupling or cycloaddition? Mendeleev Commun 28:20–21
Becke AD (1993) A new mixing of hartree-fock and local density-functional theories. J Chem Phys 98:1372–1377
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 (Revision A.7). Gaussian Inc., Pittsburgh
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218
Schlegel HB (2000) Perspective on “Ab initio calculation of force constants and equilibrium geometries in polyatomic molecules. I. Theory.” Theor Chem Acc 103:294–296
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Calais J-L (1993) Density-functional theory of atoms and molecules R.G. Parr and W. Yang, Oxford University Press, New York, Oxford, 1989. IX + 333 pp. Price £45.00: Book Review. Int J Quantum Chem 47:101–101
Day OW, Smith DW, Garrod C (2009) A generalization of the hartree-fock one-particle potential. Int J Quantum Chem 8:501–509
Morrell MM (1975) Calculation of ionization potentials from density matrices and natural functions, and the long-range behavior of natural orbitals and electron density. J Chem Phys 62:549
Cioslowski J, Piskorz P, Liu G (1997) Ionization potentials and electron affinities from the extended Koopmans’ theorem applied to energy-derivative density matrices: The EKTMPn and EKTQCISD methods. J Chem Phys 107:6804–6811
Parr RG, Pearson RG (1983) Companion parameter to absolute electronegativity. J Am Chemi Soc 105:7512–7516
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Domingo LR, Picher MT (2004) A DFT study of the Huisgen 1,3-dipolar cycloaddition between hindered thiocarbonyl ylides and tetracyanoethylene. Tetrahedron 60:5053–5058
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the local electrophilicity of organic molecules. understanding the regioselectivity on Diels−Alder reactions. J Phys Chem A 106:6871–6875
Pérez P, Domingo LR, Duque-Noreña M, Chamorro E (2009) A condensed-to-atom nucleophilicity index. an application to the director effects on the electrophilic aromatic substitutions. J of Mol Struct: THEOCHEM 895:86–91
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423
Marakchi K, Ghailane R, Kabbaj OK, Komiha N (2014) DFT study of the mechanism and stereoselectivity of the 1,3-dipolar cycloaddition between pyrroline-1-oxide and methyl crotonate. J Chem Sci 126:283–292
Benchouk W, Mekelleche SM (2008) Theoretical analysis of the regioselectivity of 1,3-dipolar cycloaddition of C-(methoxycarbonyl)-N-methyl with methyl acrylate and vinyl acetate. J f Mol Struct: THEOCHEM 852:46–53
Emamian SR, Ali-Asgari S, Zahedi E (2014) Mechanism and regioselectivity of 1,3-dipolar cycloaddition reactions of sulphur-centred dipoles with furan-2,3-dione: A theoretical study using DFT. J Chem Sci 126:293–302
Salah M, Komiha N, Kabbaj OK, Ghailane R, Marakchi K (2017) Computational study of the 1,3-dipolar cycloaddition between methyl 2-trifluorobutynoate and substituted azides in terms of reactivity indices and activation parameters. J Mol Graph Model 73:143–151
Yepes D, Valenzuela J, Martínez-Araya JI (2019) Effect of the exchange–correlation functional on the synchronicity/nonsynchronicity in bond formation in Diels-Alder reactions: a reaction force constant analysis. Phys Chem Chem Phys 21:7412–7428
Alder K, Stein G (1935) Über den sterischen Verlauf von Additions- und Substitutions-reaktionen. V. Der sterische Verlauf der Phenylazid-reaktion. Justus Liebigs Ann Chem 515:185–200
Yepes D, Murray JS, Pérez P (2014) Complementarity of reaction force and electron localization function analyses of asynchronicity in bond formation in Diels-Alder reactions. Phys Chem Chem Phys 16:6726
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428
Domingo LR, Sáez JA (2009) Understanding the mechanism of polar Diels-Alder reactions. Org Biomol Chem 7:3576–3583
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahrane, M., Marakchi, K. & Ghailane, R. Theoretical study of the Diels–Alder reaction of 3-bromo-1-phenylprop-2-ynone with furan and 2-methylfuran. Theor Chem Acc 140, 108 (2021). https://doi.org/10.1007/s00214-021-02812-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02812-2