Abstract
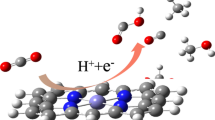
Designing highly efficient electrocatalysts for electrochemical reduction of CO2 (ERC) to value-added chemicals is a better approach to balance carbon emission. In this paper, through density functional theory (DFT) calculation, we study the catalytic performance of the catalyst supported on nitrogen-doped carbon (NC) for the electrochemical reduction of CO2. We investigated anchoring a single transition metal atom on the NC, denoted as M@d-NC (M=Rh, Ir). The results show that Rh@d-NC shows the best performance in terms of both activity and stability, which is very favorable for methane production (* + CO2 + 8H+ → C*OOH + 7H+ → CO* + 6H+ → CHO* + 5H+ → CH2O* + 4H+ → CH3O* + 3H+ → CH3OH* + 2H+ → CH3* + H+ → * + CH4). By using the microkinetic model, the rate constants of CHO* → CH2O* on Rh@d-NC and CHO* → *CHOH on Ir@d-NC are obtained. It is confirmed that Rh@d-NC has high activity and selectivity for methane formation. These findings provide design guidelines for developing efficient carbon-based catalysts that could potentially extend ERC to fuels and chemicals.









Similar content being viewed by others
References
Hirunsit P (2013) Electroreduction of carbon dioxide to methane on copper, copper-silver, and copper-gold catalysts: a dft study. J Phys Chem C 117:8262–8268
Pachauri RK, Meyer LA (2014) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change IPCC. Climate Change Synthesis Report, 151
Birdja YY, Shen J, Koper MTM (2017) Influence of the metal center of metalloprotoporphyrins on the electrocatalytic CO2 reduction to formic acid. Catal Today 288:37–47
Kuhl KP, Hatsukade T, Cave ER, Abram DN, Kibsgaard J, Jaramillo TF (2014) Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J Am Chem Soc 136:14107–14113
Lu Q, Rosen J, Jiao F (2015) Nanostructured Metallic Electrocatalysts for carbon dioxide reduction. ChemCatChem 7:38–47
Goeppert A, Czaun M, Jones JP, Surya Prakash GK, Olah GA (2014) Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem Soc Rev 43:7995–8048
Jiang K, Kharel P, Peng Y, Gangishetty MK, Lin H-YG, Stavitski E, Attenkofer K, Wang H (2017) Silver nanoparticles with surface-bonded oxygen for highly selective CO2 reduction. ACS Sustain Chem Eng 5:8529–8534
Siahrostami S, Jiang K, Karamad M, Chan K, Wang H, Nørskov J (2017) Theoretical investigations into defected graphene for electrochemical reduction of CO2. ACS Sustain Chem Eng 5:11080–11085
Jhong H-RM, Ma S, Kenis PJA (2013) Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr Opin Chem Eng 2:191–199
Costentin C, Robert M, Saveant J-M (2013) Catalysis of the electrochemical reduction of carbon dioxide. Chem Soc Rev 42:2423–2436
Wang W, Wang S, Ma X, Gong J (2011) Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev 40:3703–3727
Qiao J, Liu Y, Hong F, Zhang J (2014) A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem Soc Rev 43:631–675
Zhu DD, Liu JL, Qiao SZ (2016) Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv Mater 28:3423–3452
Benson EE, Kubiak CP, Sathrum AJ, Smieja JM (2009) Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem Soc Rev 38:89–99
Hori Y (2008) Electrochemical CO2 reduction on metal electrodes. Modern Aspects of Electrochem 42:89–189
Zhu W et al (2013) Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J Am Chem Soc 135:16833–16836
Zhu W et al (2014) Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J Am Chem Soc 136:16132–16135
Mistry H et al (2014) Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J Am Chem Soc 136:16473–16476
Lu Q, Rosen J, Zhou Y, Hutchings GS, Kimmel YC, Chen JG, Jiao F (2014) A selective and efficient electrocatalyst for carbon dioxide reduction. Nat Commun 5(1):1–6
Kim C et al (2015) Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J Am Chem Soc 137:13844–13850
Kim C et al (2017) Insight into electrochemical CO2 reduction on surface-molecule-mediated Ag nanoparticles. ACS Catal 7:779–785
Rosen J et al (2015) Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal 5:4293–4299
Hori Y, Murata A, Takahashi R (1989) Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J Chem Soc Faraday Trans 1(85):2309–2326
Peterson AA, Abild-Pedersen F, Studt F, Rossmeisl J, Norskov JK (2010) How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ Sci 3:1311–1315
Pan F, Deng W, Justiniano C, Li Y (2018) Identification of champion transition metals centers in metal and nitrogen-codoped carbon catalysts for CO2 reduction. Appl Catal B-Environ 226:463–472
Jia M, Choi C, Wu TS, Ma C, Kang P, Tao H, Fan Q, Hong S, Liu S, Soo Y-L, Jung Y, Qiu J, Sun Z (2018) Carbonsupported Ni nanoparticles for efficient CO2 electroreduction. Chem Sci 9:8775–8780
Matsubu JC, Zhang S, DeRita L, Marinkovic NS, Chen JG, Graham GW, Pan X, Christopher P (2017) Adsorbate-mediated strong metal-support interactions in oxide-supported Rh catalysts. Nat Chem 9:120–127
Back S, Lim J, Kim N-Y, Kim Y-H, Jung Y (2017) Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem Sci 8:1090–1096
Duan X, Xu J, Wei Z, Ma J, Guo S, Wang S, Dou S (2017) Metal-free carbon materials for CO2 electrochemical reduction. Adv Mater 29(41):1701784
Wu J, Yadav RM, Liu M, Sharma PP, Tiwary CS, Ma L, Zou X, Zhou X-D, Yakobson BI, Lou J, Ajayan PM (2015) Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes ACS. NANO 9:5364–5371
Kumar B, Asadi M, Pisasale D, Sinha-Ray S, Rosen BA, Haasch R, Salehi-Khojin A (2013) Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat Commun 4(1):1–8
Wu J, Liu M, Sharma PP, Yadav RM, Ma L, Yang Y, Zou X, Zhou XD, Vajtai R, Yakobson BI, Lou J, Ajayan PM (2016) Incorporation of nitrogen defects for efficient reduction of CO2 Via two-electron pathway on three-dimensional graphene foam. Nano Lett 16:466–470
Song Y, Chen W, Zhao C, Li S, Wei W, Sun Y (2017) Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol. Angew Chem Int Ed 56:10840–10844
Moller T, Ju W, Bagger A, Wang X, Luo F, Thanh TN, Varela AS, Rossmeisl J, Strasser P (2019) Efficient CO2 to CO electrolysis on solid Ni–N–C catalysts at industrial current densities. Energy Environ Sci 12:640–647
Zhao C, Dai X, Yao T, Chen W, Wang X, Wang J, Yang J, Wei S, Wu Y, Li Y (2017) Ionic Exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J Am Chem Soc 139:8078–8081
Li X, Bi W, Chen M, Sun Y, Ju H, Yan W, Zhu J, Wu X, Chu W, Wu C, Xie Y (2017) Exclusive Ni−N4 sites realize nearunity CO selectivity for electrochemical CO2 reduction. J Am Chem Soc 139:14889–14892
Yang H, Hung S, Liu S, Yuan K, Miao S, Zhang L, Huang X, Wang H, Cai W, Chen R, Gao J, Yang X, Chen W, Huang Y, Chen H, Li C, Zhang T, Liu B (2018) Atomically dispersed Ni (I) as the active site for electrochemical CO2 reduction. Nat Energy 3:140–147
Jiang K, Siahrostami S, Zheng T, Hu Y, Hwang S, Stavitski E, Peng Y, Dynes J, Gangisetty M, Su D, Attenkofer K, Wang H (2018) Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ Sci 11:893–903
Zhang A, He R, Li H, Chen Y, Kong T, Li K, Ju H, Zhu J, Zhu W, Zeng J (2018) Nickel doping in atomically thin tin disulfide nanosheets enables highly efficient CO2 reduction. Angew Chem Int Ed 57:10954–10958
Zheng T, Jiang K, Ta N, Hu Y, Zeng J, Liu J, Wang H (2019) Large-Scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 3(1):265–278
Yang F, Song P, Liu X, Mei B, Xing W, Jiang Z, Gu L, Xu W (2018) Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst. Angew Chem Int Ed 57:12303–12307
Cheng Y, Zhao S, Johannessen B, Veder JP, Saunders M, Rowles MR, Jiang SP (2018) Atomically dispersed transition metals on carbon nanotubes with ultrahigh loading for selective electrochemical carbon dioxide reduction. Adv Mater 30(13):1706287
Ye Y, Cai F, Li H, Wu H, Wang G, Li Y, Miao S, Xie S, Si R, Wang J, Bao X (2017) Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction. Nano Energy 38:281–289
Varela AS, Ju W, Bagger A, Franco P, Rossmeisl J, Strasser P (2019) Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts. ACS Catal 9:7270–7284
Pan FP, Zhang HG, Liu KX, Cullen D, More K, Wang MY, Feng ZX, Wang GF, Wu G, Li Y (2018) Unveiling active sites of CO2 reduction on nitrogen-coordinated and atomically dispersed iron and cobalt catalysts. ACS Catal 8:3116–3122
Wang ZX, Zhao JX, Cai QH (2017) CO2 electroreduction performance of a single transition metal atom supported on porphyrin-like graphene: a computational study. Phys Chem Chem Phys 19:23113–23121
Nørskov JK, Bligaard T, Rossmeisl J, Christensen CH (2009) Towards the computational design of solid catalysts. Nat Chem 1:37–46
Greeley J, Jaramillo TF, Bonde J, Chorkendorff I, Nørskov JK (2006) Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat Mater 5:909–913
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone, V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralt JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev V, Austin AJ, Cammi R, Pomelli C, Ochterski J, Martin, RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian Inc., Wallingford CT, Gaussian 09, Revision D.01
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. potentials for the transition metal atoms Sc to Hg. J Chem Phys 82(1):270–283
Peterson AA, Nørskov JK (2012) Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J Phys Chem Lett 3:251–258
Karamad M, Hansen HA, Rossmeisl J, Nørskov JK (2015) Mechanistic pathway in the electrochemical reduction of CO2 on RuO2 ACS. Catal 5:4075–4081
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure: iv constants of diatomic molecules New York:Van Nostrand Reinhold Company.
Dyer MS, Robin A, Haq S, Raval R, Persson M, Klimes J (2011) Understanding the interaction of the porphyrin macrocycle to reactive metal substrates: structure, bonding, and adatom capture. ACS Nano 5:1831–1838
Hanke F, Haq S, Raval R, Persson M (2011) Heat-to-connect: surface commensurability directs organometallic one-dimensional self-assembly. ACS Nano 5:9093–9103
Haq S, Hanke F, Dyer MS, Persson M, Iavicoli P, Amabilino DB, Raval R (2011) Clean coupling of unfunctionalized porphyrins at surfaces to give highly oriented organometallic oligomers. J Am Chem Soc 133:12031–12039
Bischoff F, Seufert K, Auwarter W, Joshi S, Vijayaraghavan S, Ecija D, Diller K, Papageorgiou AC, Fischer S, Allegretti F, Duncan DA, Klappenberger F, Blobner F, Han R, Barth JV (2013) How surface bonding and repulsive interactions cause phase transformations ordering of a prototype macrocyclic compound on Ag(111). ACS Nano 7:3139–3149
Diller K, Klappenberger F, Allegretti F, Papageorgiou AC, Fischer S, Wiengarten A, Joshi S, Seufert K, Ecija D (2013) Investigating the molecule-substrate interaction of prototypic tetrapyrrole compounds Adsorption and self-metalation of porphine on Cu(111). J Chem Phys 138:154710–1–154719
Shi C, Hansen HA, Lausche AC, Nørskov JK (2014) Trends in electrochemical CO2 reduction activity for open and close-packed metal surfaces. Phys Chem Chem Phys 16:4720–4727
Bligaard T, Nørskov JK, Dahl S, Matthiesen J, Christensen CH, Sehested J (2004) The brønsted-evans-polanyi relation and the volcano curve in heterogeneous catalysis. J Catal 224:206–217
Plessow PN, Abild-Pedersen F (2015) Examining the linearity of transition state scaling relations. J Phys Chem C 119:10448–10453
Hori Y, Wakebe H, Tsukamoto T, Koga O (1994) Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim Acta 39:1833–1839
Shen J, Kortlever R, Kas R, Birdja YY, Diaz-Morales O, Kwon Y, Koper MT (2015) Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat Commun 6(1):1–8
Ju W, Bagger A, Hao GP, Varela AS, Sinev I, Bon V, Strasser P (2017) Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO 2. Nat Commun 8(1):1–9
Hussain J, Jonsson H, Skulason E (2018) Calculations of product selectivity in electrochemical CO2 reduction. ACS Cata 8:5240–5249
Back S, Kim H, Jung Y (2015) Selective heterogeneous CO2 electroreduction to methanol. ACS Catal 5:965–971
(a) Li H, Li Y, Koper MTM, Calle-Vallejo F (2014) Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis J. Am. Chem. Soc, 136, 15694−15701. (b) Michalsky R, Zhang YJ, Peterson AA (2014) Trends in the hydrogen evolution activity of metal carbide catalysts ACS. Catal, 4, 1274−1278. (c) Klaus S, Cai Y, Louie, MW, Trotochaud L, Bell, AT (2015) Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity J. Phys. Chem. C, 119, 7243−7254. (d) Swierk J, Klaus S, Trotochaud L, Bell AT, Tilley TD (2015) Electrochemical study of the energetics of the oxygen evolution reaction at Nickel Iron (Oxy)hydroxide catalysts J. Phys. Chem. C, 119, 19022−19029
Chan K, Tsai C, Hansen HA, Nørskov JK (2014) Molybdenum sulfides and selenides as possible electrocatalysts for CO2 reduction. ChemCatChem 6:1899–1905
Bagger A, Ju W, Varela AS, Strasser P, Rossmeisl J (2017) Electrochemical CO2 reduction: a classification problem. Chem Phys Chem 18:3266–3273
Bagger A, Ju W, Varela AS, Strasser P, Rossmeisl J (2017) Single site porphyrine-like structures advantages over metals for selective electrochemical CO2 reduction. Catal Today 288:74–78
Koga O, Matsuo T, Yamazaki H, Hori Y (1998) Infrared spectroscopic observation of intermediate species on Ni and Fe electrodes in the electrochemical reduction of CO2 and CO to hydrocarbons. Bull Chem Soc Jpn 71:315–320
Nikolic BZ, Huang H, Gervasio D, Lin A, Fierro C, Adzic RR, Yeager E (1990) Electroreduction of carbon dioxide on platinum single crystal electrodes: electrochemical and in situ FTIR studies. J Electroanal Chem Interfacial Electrochem 295:415–423
Oda I, Ogasawara H, Ito M (1996) Carbon monoxide adsorption on copper and silver electrodes during carbon dioxide electroreduction studied by infrared reflection absorption spectroscopy and surface-enhanced raman spectroscopy. Langmuir 12:1094–1097
Perez-Gallent E, Figueiredo MC, Calle-Vallejo F, Koper MTM (2017) Spectroscopic observation of a hydrogenated CO dimer intermediate during CO reduction on Cu(100) electrodes. Angew Chem Int Ed 56:3621–3624
Shi C, Chan K, Yoo JS, Nørskov JK (2016) Barriers of electrochemical CO2 reduction on transition metals. Org Process Res Dev 20:1424–1430
Dewulf DW, Jin T, Bard AJ (1989) Electrochemical and surface studies of carbon dioxide reduction to methane and ethylene at copper electrodes in aqueous solutions. J Electrochem Soc 136:1686–1691
Hori Y, Takahashi R, Yoshinami Y, Murata A (1997) Electrochemical reduction of CO at a copper electrode. J Phys Chem B 101:7075–7081
Hori Y, Murata A, Takahashi R, Suzuki S (1987) Electroreduction of CO to CH4 and C2H4 at a copper electrode in aqueous-solutions at ambient-temperature and pressure. J Am Chem Soc 109:5022–5023
Kim JJ, Summers DP, Frese KW Jr (1988) Reduction of carbon dioxide and carbon monoxide to methane on copper foil electrodes. J Electroanal Chem Interfacial Electrochem 245:223–244
Filot IAW, Zijlstra B, Hensen EJM (2019) http://www.mkmcxx.nl (accessed Dec 2, 2019).
Zhang X, Li Y, Guo P, Le J-B, Zhou Z-Y, Cheng J, Sun S-G (2019) Theory on optimizing the activity of electrocatalytic proton coupled electron transfer reactions. J Catal 376:17–24
Lu XQ, Deng ZG, Wei SX, Zhu Q, Wang WL, Guo WY, Wu CML (2015) CO tolerance of a Pt3Sn(111) catalyst in ethanol decomposition. Catal Sci Technol 5:3246–3258
Liu P, Rodriguez JA (2006) Water-Gas-Shift reaction on molybdenum carbide surfaces: essential role of the oxycarbide. J Phys Chem B 110:19418–19425
Vilekar SA, Fishtik I, Datta R (2010) Kinetics of the hydrogen electrode reaction. J Electrochem Soc 157:B1040–B1050
Hammer B, Nørskov JK (2000) Theoretical surface science and catalysis-calculations and concepts. Adv Catal 31:71–129
Lima FHB, Zhang J, Shao MH, Sasaki K, Vukmirovic MB, Ticianelli EA, Adzic RR (2007) Catalytic activity-d-Band center correlation for the O2 reduction reaction on platinum in alkaline solutions. J Phys Chem C 111:404–410
Hammer B, Nørskov JK (1995) Why gold is the noblest of all the metals. Nature 376:238–240
Acknowledgements
This work was financially supported by Undergraduate Training Programs for Innovation and Entrepreneurship of Shanxi Province and Shanxi Medical University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, S. CO2 electrochemical reduction to methane on transition metal porphyrin nitrogen-doped carbon material M@d-NC: theoretical insight. Theor Chem Acc 140, 78 (2021). https://doi.org/10.1007/s00214-021-02788-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02788-z