Abstract
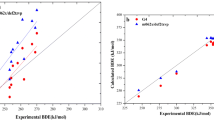
Hydrogen atom transfer is one important reaction in biological system, in industry, and in atmosphere. The reaction is preluded by hydrogen bond dissociation. To gain a comprehensive understanding on the reaction, it is necessary to investigate how the current computational methods model hydrogen bond dissociation. As a starting point, we utilized density functional theory-based calculations to identify the effect of dispersion and long-range corrections on O—H and C—H dissociations in non-phenyl and phenyl groups. We employed five different methods, namely B3LYP, CAM-B3LYP (with long-range correction), M06-2X, and B3LYP and CAM-B3LYP with the D3 version of Grimme’s dispersion. The results showed that for the case of O—H dissociation in two member of phenyl groups, namely phenol and catechol, the dispersion correction’s effect was negligible, but the long-range correction’s effect was significant. The significant effect was shown by the increasing of energy barrier and the shortening of O—H interatomic distance in the transition state. Therefore, we suggest one should consider the long-range correction in modeling hydrogen bond dissociation in phenolic compounds, namely phenol and catechol.




Similar content being viewed by others
References
Zielinski ZAM, Pratt DA (2017) J Org Chem 82(6):2817–2825
Yin H, Porter NA (2011) Chem Rev 111(10):5944–5972
Shang Y, Zhou H, Li X, Zhou J, Chen K (2019) New J Chem 43:15736–15742
Vo QV, Nam PC, Bay MV, Thong NM, Cuong ND, Mechler A (2018) Sci Rep 8:12361
Xue Y, Zheng Y, An L, Dou Y, Liu Y (2014) Food Chem 151:198–206
Iuga C, Alvarez-Idaboy JR, Russo N (2012) J Org Chem 12:3868–3877
Galano A, Alvarez-Diduk R, Ramirez-Silva MT, Alarcon-Angeles G, Rojas-Hernandez A (2009) Chem Phys 363:13–23
Jovanovic SV, Steenken S, Boone CW, Simic MG (1999) J Am Chem Soc 121:9677–9681
Wang Y-N, Eriksson LA (2001) Theor Chem Acc 106:158–162
Mallick S, Sarkar S, Bandyopadhyay B, Kumar P (2018) J Phys Chem A 122(1):350–363
Kumar M, Sinha A, Francisco JS (2016) Acc Chem Res 49(5):877–883
Liang F, Zhong W, Xiang L, Mao L, Xu Q, Kirk SR, Yin D (2019) J Catal 378:256–269
Asgari P, Hua Y, Bokka A, Thiamsiri C, Prasitwatcharakorn W, Karedath A, Chen X, Sardar S, Yum K, Leem G, Pierce BS, Nam K, Gao J, Jeon J (2019) Nat Catal 2:164–173
Barckholtz C, Barckholtz TA, Hadad CM (1999) J Am Chem Soc 121(3):491–500
Wang L, Yang F, Zhao X, Li Y (2019) Food Chem 275:339–345
Nantasenamat C, Isarankura-Na-Ayudhya C, Naenna T, Prachayasittikul V (2008) J Mol Graph Model 27:188–196
Zhang H-Y, Sun Y-M, Wang X-L (2003) Chem Eur J 9:502–508
Brinck T, Lee H-N, Jonsson M (1999) J Phys Chem 103:7094–7104
Izgorodina EI, Coote ML, Radom L (2005) J Phys Chem A 109:7558–7566
Izgorodina EI, Brittain DRB, Hodgson JL, Krenske EH, Lin CY, Namazian M, Coote ML (2007) J Phys Chem A 111:10754–10768
Zhao Y, Truhlar DG (2008) J Phys Chem A 112:1095–1099
Beste A, Buchanan AC III (2009) J Org Chem 74(7):2837–2841
Zheng Y-Z, Fu Z-M, Deng G, Guo R, Chen D-F (2020) Phytochemistry 178:112454
Du T, Quina FH, Tunega D, Zhang J, Aquino AJA (2020) Theor Chem Acc 139:75
Yanai T, Tew DP, Handy NC (2004) Chem Phys Lett 393:51–57
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Becke AD (1993) J Chem Phys 98:5648
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Rusydi F, Madinah R, Puspitasari I, Mark-Lee WF, Ahmad A, Rusydi A (2020). Biochem Mol Biol Educ. https://doi.org/10.1002/bmb.21433
Fadilla RN, Rusydi F, Aisyah ND, Khoirunisa V, Dipojono HK, Ahmad F, Mudasir, Puspitasari I (2020) Molecules 25: 670
Rusydi F, Aisyah ND, Fadilla RN, Dipojono HK, Ahmad F, Mudasir, Puspitasari I, Rusydi A (2019) Heliyon 5: e02409
Fadilla RN, Aisyah ND, Dipojono HK, Rusydi F (2017) Procedia Eng 170:113–118
Hohenberg P, Kohn W (1964) Phys Rev 136:B864
Kohn W, Sham LJ (1965) Phys Rev 140:A1133
Frisch MJ, Trucks GW, Schlegel HB,Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V,Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV,Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D,Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A,Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng, Liang W, Hada M, Ehara M, Toyota K, Fukuda R,Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H,Vreven T, Throssell K, Montgomery JA, Jr., Peralta J E,Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin K N, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2013) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT
Glendening ED, Reed AE, Carpenter JE, Weinhold F Nbo version 3.1
Mardirossian N, Head-Gordon M (2016) J Chem Theory Comput 12:4303–4325
Jones DB, da Silva GB, Neves RFC, Duque HV, Chiari L, de Oliveira EM, Lopes MCA, da Costa RF, Varella MTN, Bettega MHF, Lima MAP, Brunger MJ (2014) J Chem Phys 141:074314
Huber KP, Herzberg G (1979) Molecular spectra and moelcular structure IV constants of diatomic molecules. Springer, US, p 508
Young DC, Computational chemistry: A practical guide for applying techniques to real-world problems. Wiley, New York, 2001, Chp. 16, Page 138
Haynes WM (2014) CRC Handbook of Chemistry and Physics, 95th ed., CRC Press, Boca Rotan, Chp.9
Lucarini M, Pedulli GF, Guerra M (2004) Chem Eur J 10:933–939
Kamiya M, Tsuneda T, Hirao K (2002) J Chem Phys 117:6010
Parker K, Davis SR (1999) J Am Chem Soc 121:4271–4277
Zhu L, Bozzelli JW (2003) J Phys Chem A 107:3696–3703
Altarawneh M, Dlugogorski BZ, Kennedy EM, Mackie J (2010) J Phys Chem A 114:1060–1067
Chan B, Morris M, Radom L (2011) Aust J Chem 64:394–402
Peach MJG, Helgaker T, Salek P, Keal TW, Lutnæs OB, Tozer DJ, Handyd NC (2006) Phys Chem Chem Phys 8:558–562
Acknowledgements
Authors thank to Rizka Nur Fadilla (Universitas Airlangga, Indonesia) and Prof. Azizan Ahmad (University Kebangsaan Malaysia, Malaysia) for the insightful discussions. LSPB is grateful for the doctoral scholarship by Lembaga Pengelola Dana Pendidikan (LPDP). All calculations using Gaussian 16 software are performed at Riven Cluster, the high-performance computing facility in Research Center for Quantum Engineering Design, Universitas Airlangga, Indonesia.
Author information
Authors and Affiliations
Contributions
F.R. contributed to conceptualization; L.S.P.B, H.R., and I.P. contributed to formal analysis; L.S.P.B and V.K. were involved in investigation; F.R. and L.S.P.B contributed to methodology; I.P. provided the resources; L.S.P.B contributed to writing—original draft preparation; F.R. and H.K.D contributed to writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by Universitas Airlangga under grant scheme Riset Kolaborasi Mitra Luar Negeri 2019 no. 1148/UN3.14/LT/2019 and by Direktorat Riset dan Pengabdian Masyarakat, Deputi Bidang Penguatan Riset dan Pengembangan Kementerian Riset dan Teknologi/Badan Riset dan Inovasi Nasional, Republik Indonesia under grant scheme Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) 2020 no. 1288r/I1.C06/PL/2020.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Availability of Data and Materials
All data analyzed during this study are included in this published article and its supplementary information file.
Code Availability
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pulo Boli, L.S., Rusydi, F., Khoirunisa, V. et al. O—H and C—H bond dissociations in non-phenyl and phenyl groups: A DFT study with dispersion and long-range corrections. Theor Chem Acc 140, 94 (2021). https://doi.org/10.1007/s00214-021-02781-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02781-6