Abstract
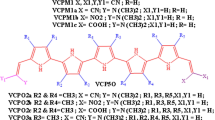
The furan oligomer with 1π-bridge to 5π-bridge has been appraised for optoelectronic properties, using Density Functional Theory and Time Dependent Density Functional Theory (DFT/TDDFT) methods. Frontier Molecular Orbital analysis aids in band gap calculation of furan oligomers and limelight the Highest Occupied Molecular Orbitals (HOMOs) lobes localized on π-bridge and donor & LUMOs are localized on π-bridge and acceptor moiety. Values of common aromaticity indices include Harmonic Oscillator Model of Aromaticity (HOMA) and Bird’s (Aromaticity Index) AI for electronic delocalization indicates high magnitude of aromaticity at acceptor end in furan oligomers, and also the magnitude increases with the increase in the number of π-bridges. The hyperpolarizability (β◦) values show many-fold degree of enhancement in its magnitude by the addition of π-bridge. The absorption maxima of furan oligomers in gas and Dichloromethane medium (DCM) are calculated through TDDFT calculations. Polarizable continuum model (PCM) analysis demonstrates that furan oligomer shows positive solvatochromism. Natural Bond Orbital analysis helps to sustain the evidence for delocalization in these systems. Overall the furan oligomer with more π-bridge shows, an enhanced aromaticity through electronic delocalization which is very helpful to enhance optoelectronic property and anti-corrosion ability.






Similar content being viewed by others
References
Reynolds J, Skotheim TA (2007) Handbook of conducting polymers, 3rd edn. CRC Press, Boca Raton
Mullen K, Wegner G (eds) (1998) Electronic materials: the oligomer approach. Wiley, Weinheim
Sariciftci NS, Smilowitz L, Heeger AJ, Wudl F (1992) Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 258:1474
Katz HE, Bao ZJ (2000) The physical chemistry of organic field-effect transistors. Phys Chem B 104:671–678
Facchetti A (2007) Semiconductors for organic transistors. Mater Today 10:28–37
Zhang L, Colella NS, Cherniawski BP, Mannsfeld SC, Briseno AL (2014) Oligothiophene semiconductors: synthesis characterization and applications for organic devices. ACS Appl Mater Interfaces 6:5327–5343
Holcombe TW, Norton JE, Rivnay J, Woo CH, Goris L, Piliego C, Griffini G, Sellinger A, Bredas JL, Salleo A, Frechet JM (2011) Steric control of the donor/acceptor Interface: implications in organic photovoltaic charge generation. J Am Chem Soc 133:12106–12114
Beaujuge PM, Amb CM, Reynolds JR (2010) Spectral engineering in π-conjugated polymers with intramolecular donor-acceptor interactions. Acc Chem Res 44:1396–1407
Wang S, Oldham WJ Jr, Hudack RA Jr, Bazan GC (2000) Synthesis morphology and optical properties of Tetrahedral Oligo (phenylenevinylene). Mater J Chem Soc. 122:5695–5709
Gidron O, Diskin-Posner Y, Bendikov M (2010) α Oligofurans. J Am Chem Soc 132:2148–4150
Perepichka IF, Perepichk DF (2009) Handbook of thiophene-based materials. Wiley, Weinheim
Fichou D (2008) Handbook of oligo and polythiophenes. Wiley, Hoboken
Mishra A, Ma CQ, Bauerle P (2009) Functional oligothiophenes-molecular design for multidimensional nanoarchitechtures and their applications. Chem ReV 109:1141–1276
Perepichka IF, Perepichka DF, Meng H, Wudl F (2005) Light emitting polythiophenes. ADV Mater 17:2281
Bauerle P (1998) In: Electronic materials: the oligomer approach Eds. Wiley-VCH, Weinheim Germany Chapter 2
Anthony JE (2008) The larger acenes: versatile organic semiconductors agnew. Chem Int Ed 47:452–483
Chun D, Cheng Y, Wudl F (2008) The most stable and fully characterized functional heptacene. Agnew Chem Int Ed 47:8380
Kaur I, Stein NN, Kopreski RP, Miller GP (2009) Exploiting substituent effects for the synthesis of a photooxidatively resistant heptacene derivative. J Am Chem Soc 131:3424
Patra A, Wijsboom YH, Zade SS, Li M, Sheynin Y, Leitus G, Bendikov M (2008) Poly(3,4-ethylenedioxyselenophene). J Am Chem Soc. 130:6734–6736
Li M, Patra A, Sheynin Y, Bendikov M (2009) Hexyl-Derivatized Poly (3, 4-ethylenedioxyselenophene): novel highly stable organic electrochromic material with high contrast ratio high coloration efficiency. Adv Mater 21:1707–1711
Li M, Sheynin Y, Patra A, Bendikov M (2009) Tuning the electrochromic properties of poly(alkyl-3,4-ethylenedioxyselenophenes) having high contrast ratio and coloration efficiency. Chem Mater 21:2482–2488
Wijsboom YH, Patra A, Zade SS, Sheynin Y, Li M, Shimon LJW, Bendikov M (2009) Controlling rigidity and planarity in conjugated polymers: poly(3,4-ethylenedithioselenophene). Angew Chem 121:5551–5555
Gajalakshmi D, Vijay Solomon R, Tamilmani V, Boobalan M, Venuvanalingam P (2015) A DFT/TDDFT mission to probe push pull vinyl coupled thiophene oligomers for optoelectronic applications. RSC Adv 5:50353–50364
Gajalakshmi D, Boobalan M, Vijay Solomon R, Tamilmani V (2019) Are vinyl coupled Furan derivatives better than thiophene derivatives for optoelectronic applications?-Answers from DFT/TDDFT calculations. J commatsci 162:60–68
Gajalakshmi D, Tamilmani V (2016) Unraveling the role of π–conjugation in thiophene oligomer for optoelectronic properties by DFT/TDDFT approach. Braz Arch Biol Technol 59(2):1–14
Becke AD (1993) Density functional thermochemistry III the role of exact exchange. J Chem Phys 98(7):5648–5652
Lee W, Yang R, Parr G (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum A direct utilization of Ab initio molecular potentials for the prevision of solvent effects. J Chem Phys 55:117–129
Zhao Y, Truhlar DG (2008) Density functional with broad applicability in chemistry. Acc Chem Res 41:157–1679
Bader RWF (1990) Atoms in molecules a quantum theory calendon Press, Oxford, R.F.W. Bader. Acc Chem Res. 18 (1985): 9–15
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1998) NBO version 3.1
Reed AE, Curtiss LA, Weinhold F (1988) Intramolecular interactions from a natural bond orbitals donor-acceptor view point. Chem Rev 8:899–926
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD,Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko, A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 (Revision A.7) Gaussian, Inc., Pittsburgh
Calvo-Losada S, Quirante Sánchez JJ (2008) pericyclic versus pseudo pericyclic reaction. What the laplacian of charge density delsquare rho(r) has to say about it? The case of cycloaddition reactions. J Phys Chem A 112:8164–8178
Aikens CM, Li S, Schatz GC (2008) From discrete electronic states to plasmons: TDDFT optical absorption properties of Agn (n=10, 20, 35, 56, 84, 120) tetrahedral Clusters. J Phys Chem Int C 112:11272–11279
Gross EKU, Kohn W (1990) Time-dependent density-functional theory. Adv Quant Chem 21:255–291
Kulhanek J, Bures F, Wojciechowski A, Makowska-Janusik M, Gondek E, Kityk I (2010) Optical operation by chromophores featuring 4,5-dicyanoimidazole embedded within poly(methyl methacrylate) matrices. J Phys Chem A. 114(35):9440–9446
Makowska-Janusik M, Kityk I, Kulhanek J, Bure F (2011) Peculiarities of the environmental influence of the optical properties of push-pull nonlinear optical molecules: a theoretical study. J Phys Chem A 115(44):12251–12258
Aittala PJ, Cramariuc O, Hukka TI, Vasilescu M, Bandula R, Lemmetyinen H (2010) A TDDFT study of the fluorescence properties of three Alkoxypyridylindolizine derivatives. J Phys Chem A 114:7094–7101
Peach MJG, Le Sueur CR, Ruud K, Guillaume M, Tozer DJ (2009) TDDFT diagnostic testing and functional assessment for triazene chromophores. Phys Chem Chem Phys 11:4465–4470
Petsalakis I, Georgiadou D, Vasilopoulou M, Pistolis G, Dimotikali D, Argitis P, Theodorakopoulos G (2010) Theoretical investigation on the effect of protonation on the absorption and emission spectra of two amine-group-bearing, red push pull emitters, 4-Dimethylamino-4-nitrostilbene and 4-(dicyanomethylene)-2-methyl-6-p-(dimethylamino) styryl-4H-pyran, by DFT and TDDFT calculations. J Phys Chem A 114:5580–5587
Fitzner R, Mena-Osteritz E, Mishra A, Schulz G, Reinold E, Weil M, Körner C, Ziehlke H, Elschner C, Leo K, Riede M, Pfeiffer M, Uhrich C, Bäuerle P (2012) correlation of π-conjugated oligomer structure with film morphology and organic solar cell performance. J Am Chem soc 134:11064–11067
Pedone A, Bloino J, Monti S, Prampolini G, Barone V (2009) Absorption and emission UV-Vis spectra of the TRITC fluorophore molecule in solution a quantum mechanical study. Phys Chem Chem Phys 12:1000–1006
Barone V, Bloino J, Monti S, Pedone A, Prampolini G (2010) Theoretical multilevel approach for studying the photophysical properties of organic dyes in solution. Phys Chem Chem Phys 12:10550–10561
Geerlings P, De Proft F (2002) Chemical reactivity as described by quantum chemical methods. Int J Moll Sci 3:276–309
Acknowledgements
The authors thank Emeritus Prof. P.Venuvanalingam, Bharathidasan University Tiruchirapalli for computational facility and fruitful discussion rendered during this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gajalakshmi, D., Tamilmani, V. & Jaccob, M. A comprehensive investigation of structural features, electron delocalization, optoelectronic and anti-corrosion characteristics in furan oligomers by DFT/TDDFT method. Theor Chem Acc 140, 81 (2021). https://doi.org/10.1007/s00214-021-02760-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02760-x