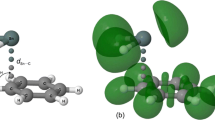
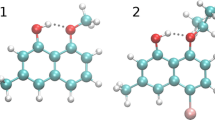
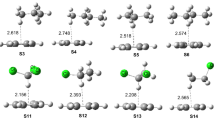
Abstract
In this article, atom-pairwise dispersion interaction terms were calculated to describe the origin of dispersion interaction in weakly bound molecular clusters. A series of C–H/π bound phenylacetylene–hydrocarbon complexes were computationally investigated, using the dispersion-corrected DFT methods B3LYP-D3(BJ) and PBE0-D3(BJ) with def2-TZVPP and aug-cc-pVDZ basis sets. The geometry-optimized structures were used to quantitatively analyze the binding energy dependence on several atomic and molecular polarizability parameters. The binding energies of the dispersively bound complexes were excellently correlated linearly with the cumulative dispersion terms (ED3(BJ) and ETD) calculated using Grimme's D3(BJ) and D2 numerical methods, respectively. Only the binary complexes with significant contributions from electrostatic terms are found to deviate from linearity. Depending on the contribution of individual atoms of the ad-molecules, dispersion-layers were proposed. The first layers of hydrogen and carbon atoms have contributed ~ 85% of the total interaction energy. In the most stable structures, the ad-molecules were positioned atop of the center of mass of the PHA molecules suggesting the maximum atom-pair contacts between the interacting partners. The current results provide proof for the atomic polarizability parameters to be accountable for the dispersion energy and do not support ‘sandwiched hydrogen atom’ and ‘C–H···π hydrogen bonding’ models for the face-bound hydrocarbon complexes. The CHn number calculated from structural parameters showed a linear correlation with the binding energy, which suggests the directionless properties of the C–H/π interaction.










Similar content being viewed by others
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Knochenmuss R, Sinha RK, Leutwyler S (2020) Benchmark experimental gas-phase intermolecular dissociation energies by the SEP-R2PI method. Annu Rev Phys Chem 71:189–211. https://doi.org/10.1146/annurev-physchem-050317-014224
Tsuzuki S, Fujii A (2008) Nature and physical origin of CH/π interaction: significant difference from conventional hydrogen bonds. Phys Chem Chem Phys 10:2584–2594. https://doi.org/10.1039/B718656H
Wagner JP, Schreiner PR (2015) London Dispersion in Molecular Chemistry—Reconsidering Steric Effects. Angew Chemie Int Ed 54:12274–12296. https://doi.org/10.1002/anie.201503476
Morita S, Fujii A, Mikami N, Tsuzuki S (2006) Origin of the attraction in aliphatic C-H/pi interactions: infrared spectroscopic and theoretical characterization of gas-phase clusters of aromatics with methane. J Phys Chem A 110:10583–10590. https://doi.org/10.1021/jp064297k
Maity S, Knochenmuss R, Holzer C et al (2016) Accurate dissociation energies of two isomers of the 1-naphtholcyclopropane complex. J Chem Phys 145:164304. https://doi.org/10.1063/1.4965821
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264. https://doi.org/10.1021/cr990050q
Frey JA, Holzer C, Klopper W, Leutwyler S (2016) Experimental and theoretical determination of dissociation energies of dispersion-dominated aromatic molecular complexes. Chem Rev 116:5614–5641. https://doi.org/10.1021/acs.chemrev.5b00652
Ran J, Wong MW (2006) Saturated hydrocarbon−benzene complexes: theoretical study of cooperative CH/π interactions. J Phys Chem A 110:9702–9709. https://doi.org/10.1021/jp0555403
Nishio M (2011) The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys Chem Chem Phys 13:13873–13900. https://doi.org/10.1039/C1CP20404A
Fujii A, Hayashi H, Park JW et al (2011) Experimental and theoretical determination of the accurate CH/π interaction energies in benzene–alkane clusters: correlation between interaction energy and polarizability. Phys Chem Chem Phys 13:14131–14141. https://doi.org/10.1039/C1CP20203K
Brandl M, Weiss MS, Jabs A et al (2001) C-hπ-interactions in proteins. J Mol Biol 307:357–377. https://doi.org/10.1006/jmbi.2000.4473
Nishio M, Umezawa Y, Fantini J et al (2014) CH-π hydrogen bonds in biological macromolecules. Phys Chem Chem Phys 16:12648–12683
Knochenmuss R, Maity S, Balmer F et al (2018) Intermolecular dissociation energies of 1-naphthol·n-alkane complexes. J Chem Phys 149:34306. https://doi.org/10.1063/1.5034110
Knochenmuss R, Sinha RK, Leutwyler S (2018) Intermolecular dissociation energies of dispersively bound complexes of aromatics with noble gases and nitrogen. J Chem Phys 148:134302. https://doi.org/10.1063/1.5019432
Maity S, Ottiger P, Balmer FA et al (2016) Intermolecular dissociation energies of dispersively bound 1-naphtholcycloalkane complexes. J Chem Phys 145:244314. https://doi.org/10.1063/1.4973013
Maity S, Patwari GN, Karthikeyan S, Kim KS (2010) Binary complexes of tertiary amines with phenylacetylene. Dispersion wins over electrostatics. Phys Chem Chem Phys 12:6150–6156. https://doi.org/10.1039/B918013C
Nishio M, Umezawa Y, Honda K et al (2009) CH/π hydrogen bonds in organic and organometallic chemistry. CrystEngComm 11:1757. https://doi.org/10.1039/b902318f
Maity S, Guin M, Singh PC, Patwari GN (2010) Phenylacetylene: a hydrogen bonding chameleon. ChemPhysChem 12:26–46. https://doi.org/10.1002/cphc.201000630
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
Knochenmuss R, Sinha RK, Balmer FA et al (2020) Intermolecular dissociation energies of 1-naphthol complexes with large dispersion-energy donors: decalins and adamantane. J Chem Phys 152:104304. https://doi.org/10.1063/1.5144773
Haldar S, Gnanasekaran R, Hobza P (2015) A comparison of ab initio quantum-mechanical and experimental D0 binding energies of eleven H-bonded and eleven dispersion-bound complexes. Phys Chem Chem Phys 17:26645–26652. https://doi.org/10.1039/C5CP04427H
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100:5829–5835. https://doi.org/10.1063/1.467146
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
London F (1930) Properties and applications of molecular forces. Z Phys Chem 11(Abt:B), 222–251
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Ulrich NW, Seifert NA, Dorris RE et al (2014) Benzene⋯acetylene: a structural investigation of the prototypical CH⋯π interaction. Phys Chem Chem Phys 16:8886–8894. https://doi.org/10.1039/C4CP00845F
NIST Computational Chemistry Comparison and Benchmark Database (2019) NIST Standard Reference Database Number 101 Release 20, August 2019, Editor: Russell D. Johnson III http://cccbdb.nist.gov/
Shibasaki K, Fujii A, Mikami N, Tsuzuki S (2007) Magnitude and nature of interactions in benzene−X (X = Ethylene and Acetylene) in the gas phase: significantly different CH/π interaction of acetylene as compared with those of ethylene and methane. J Phys Chem A 111:753–758. https://doi.org/10.1021/jp065076h
Acknowledgements
This work has been supported by the Department of Chemistry, IIT Hyderabad and MHRD, the Government of India. SK thanks the Ministry of Education, Government of India, for the research fellowship
Funding
There is no funding to report for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khodia, S., Halder, S., Sarkar, S. et al. The account of atom-pair dispersion interaction on the stabilization of C–H/π bound phenylacetylene–hydrocarbon complexes. Theor Chem Acc 140, 46 (2021). https://doi.org/10.1007/s00214-021-02757-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02757-6