Abstract
Parallel-tempering Monte Carlo calculations have been performed for the Hg\(_8\) cluster in an isothermal-isobaric ensemble. Temperature and pressure dependent cluster residual heat capacity and Pearson correlation coefficient for cluster energy and volume have been calculated over a broad range of cluster temperatures and external pressures using a 2D multiple histogram method. Calculated data have subsequently been used in investigations of structural changes in \(\mathrm {Hg}_{8}\) induced by increased pressure and temperature. A global picture of these changes is provided in the form of a cluster temperature–pressure phase diagram.






Similar content being viewed by others
Notes
The local optimizations have been performed, if not stated otherwise, on the cluster ground-state potential energy surface, \(\mathcal {E}^\mathrm {int}(\mathbf {q})\), i.e., at \(P=0\) Pa.
The Pearson correlation coefficient adopts values between −1 and +1. For \(\rho \approx +1\), the two quantities compared by it are strongly correlated, i.e., if one grows, the other also grows, and vice versa. If, on the other hand, \(\rho \approx -1\), the two quantities are anti-correlated, and if one grows, the other decreases. It means that, e.g., if two structural isomers coexist at specific conditions, one being energetically richer and having smaller volume and the other being more stable but having larger volume, the energy-volume Pearson coefficient becomes negative or even approaches \(-1\). Closeness of the energy-volume Pearson correlation coefficient to +1 may indicate, on the other hand, the coexistence of structures for which higher internal energies are accompanied by larger volumes, e.g., delocalized dissociate structures formed during evaporation events. Clearly, thermal excitations may make the picture less sharp and may move the Pearson coefficient values away from the two extremes, −1 and +1. A zero value of the Pearson coefficient indicates that the two compared quantities are uncorrelated and, in the specific case of volume and energy, it may mean that internal energies and volumes of structures recorded in course of the simulation are more or less independent of each other.
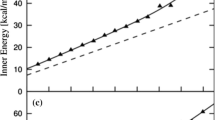
It should be noted that the features observed on the \(C_P^{\mathrm {res}}(T)\) curves are only faint and are well seen in Fig. 1 mainly because of the logarithmic scale used on the vertical axis. This particularly holds for the tiny maxima observed at low temperatures on the high-pressure curves (\(P \ge 81\,\hbox {MPa}\)).
The fall of the Pearson energy-volume correlation coefficient close to \(-1\) indicates a strong anti-correlation between the values of the cluster internal energy and its volume. It means that two families of cluster structures alternate, higher-energy structures with smaller volumes and structures with larger cluster volumes and lower energies.
Just for \(T = 11\,\hbox {K}\), and presumably also for other close temperatures, a considerable amount of higher-energy isomer 3 emerges at pressures approaching the \(P=1\) GPa limit. It should be noted, however, that this isomer disappears again at pressures above \(P\approx 1.4\) GPa (not shown in the figure) as well as at higher temperatures (represented here by \(T=61\,\hbox {K}\)).
Not speaking about the fact that evaporation plays a role at low pressures and the increased cluster temperature, in contrast to the low-temperature regime.
The only exception to this observation is the pressure-induced transition from isomer 1 to isomer 2 detected at increased pressures (\(P\approx 20\)–\(140\,\hbox {MPa}\)) and low temperatures (below \(T \approx 60\,\hbox {K}\)). As is clear from Fig. 4, this transformation is not accompanied by any specific feature in the Pearson correlation coefficient and is only marked by a well apparent maximum on the cluster heat capacity plotted as a function of pressure. However, this maximum corresponds rather to pressures for which isomer 2 has already become dominant than to the coexistence region of the observed transition. For this reason, we have represented this coexistence region by a curve resulting from a condition that the abundances of isomers 1 and 2 are equal to each other.
References
Singh PP (1994) Phys. Rev. Lett. 72:2446
Calvo F, Pahl E, Wormit M, Schwerdtfeger P (2013) Angew. Chem. Int. Ed. 52:7583
Bréchignac C, Broyer M, Cahuzac P, Delacretaz G, Labastie G, Wöste L (1985) Chem. Phys. Lett. 120:559
Bréchignac C, Broyer M, Cahuzac P, Delacretaz G, Labastie G, Wolf JP, Wöste L (1988) Phys. Rev. Lett. 60:275
Rademann K, Kaiser B, Even U, Hensel F (1987) Phys. Rev. Lett. 59:2319
Haberland H, Langosch H (1991) Z. Phys. D 19:223
Busani R, Folkers M, Cheshnovsky O (1998) Phys. Rev. Lett. 81:3836
Kitamura H (2006) Chem. Phys. 325:207
Calvo F, Pahl E, Schwerdtfeger P, Spiegelman F (2012) J. Chem. Theor. Comp. 8:639
Berry RS (1999). In: Jellinek J (ed) Theory of atomic and molecular clusters. Springer, Berlin, pp 1–26
Berry RS (2017). In: Sattler KD (ed) Handbook of nanophysics. Principles and methods. CRC Press, Cambridge, pp 20.1-20.15
Vítek A, Ofiala A, Kalus R (2012) Phys. Chem. Chem. Phys. 14:15509
Vítek A, Arismendi-Arrieta DJ, Rodríguez-Cantano R, Prosmiti R, Villarreal P, Kalus R, Delgado-Barrio G (2015) Phys. Chem. Chem. Phys. 17:8792
Šarmanová M, Vítek A, Ćosić R, Kalus R (2019) RSC Adv. 9:37258. https://doi.org/10.1039/c9ra08000g
Ellison FO (1963) J. Am. Chem. Soc. 85:3540
Kitamura H (2006) Chem. Phys. Lett. 425:205
Ćosić R, Vítek A, Kalus R (2018) Phys. Chem. Chem. Phys. 20:28871
Pahl E, Figgen D, Thierfelder C, Petterson KA, Calvo F, Schwerdtfeger P (2010) J. Chem. Phys. 132:114301
Czuchaj E, Rebentrost F, Stoll H, Preuss H (1997) Chem. Phys. 214:277
Cohen JS, Schneider BI (1974) J. Chem. Phys. 61:3230
Barber CB, Dobkin DP, Huhdanpaa H (1996) ACM Trans. Math. Softw. 22(4):469. https://doi.org/10.1145/235815.235821
Bondi A (1964) J. Phys. Chem. 68:441. https://doi.org/10.1021/j100785a001
Maibaum L, Schmidt M, Löwen H (2001) Phys. Rev. E 63:051401
Vítek A, Arismendi-Arrieta DJ, Šarmanová M, Kalus R, Prosmiti R (2020) J. Phys. Chem. A 124(20):4036. https://doi.org/10.1021/acs.jpca.0c00881
Okabe T, Kawata M, Okamoto Y, Mikami M (2001) Chem. Phys. Lett. 335:435
Vítek A, Kalus R (2014) Comput. Phys. Commun. 185(6):1595. https://doi.org/10.1016/j.cpc.2014.02.024
Pal A, Roy SK (2004) Phys. Rev. E 69:021709. https://doi.org/10.1103/PhysRevE.69.021709
Calvo F, Doye JPK, Wales DJ (2001) J. Chem. Phys. 115:9627
Acknowledgements
Financial support from the Ministry of Education, Youth, and Sports of the Czech Republic (Grants No. LM2018140 and LQ1602) and the European Union Horizon 2020 research and innovation program (Grant No. 768892) was acknowledged. The computational results have been achieved using the supercomputers of the IT4Innovations National Supercomputing Center (Grant No. OPEN-19-48)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles “Festschrift in honor of Fernand Spiegelmann”.
Rights and permissions
About this article
Cite this article
Vítek, A., Šarmanová, M. & Kalus, R. Isothermal-isobaric thermodynamics of small \(\mathrm {Hg}_{N}\) clusters. A case study on \(\mathrm {Hg}_{8}\).. Theor Chem Acc 140, 54 (2021). https://doi.org/10.1007/s00214-021-02739-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02739-8