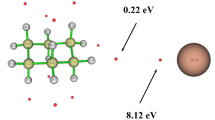
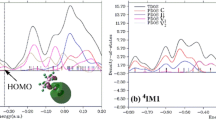
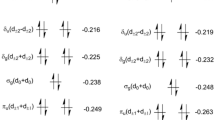Abstract
Hetero-nuclear MnNi+ cation exhibits high catalytic activity in the C–H bond activation of cyclohexane in comparison with mono-nuclear Mn+ cation. The dehydrogenation reaction mechanistic investigations of cyclohexane catalyzed by MnNi+ and Mn+ have been carried out with density functional theory calculations. The activations of the first, third and fifth C–H bond of cyclohexane occur mainly because of the orbital interaction between the C–H σ bond of cyclic hydrocarbon and the empty d orbital of Mn. The smaller energy gaps of interacting orbitals result in stronger electron transfer between the related orbitals, which can elucidate MnNi+ cation is more reactive than Mn+. The coordination bonds between MnNi+ and cycloolefin in the second and third dehydrogenation processes of cyclohexane are another important factor for accelerating the dehydrogenation, which can stabilize the transition states for the activation of C–H bonds. The lower the transition state energy, the easier the C–H bond activation.








Similar content being viewed by others
References
Arndtsen BA, Bergman RG, Mobley TA, Peterson TH (1995) Selective intermolecular carbon–hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc Chem Res 28:154–162
Crabtree RH (2001) Alkane C–H activation and functionalization with homogeneous transition metal catalysts: a century of progress—a new millennium in prospect. J Chem Soc Dalton Trans 17:2437–2450
Labinger JA, Bercaw JE (2002) Understanding and exploiting C–H bond activation. Nature 417:507–514
Shilov AE, Shul’pin GB (1997) Activation of C–H bonds by metal complexes. Chem Rev 97:2879–2932
Liégault B, Lapointe D, Caron L, Vlassova A, Fagnou K (2009) Establishment of broadly applicable reaction conditions for the palladium-catalyzed direct arylation of heteroatom-containing aromatic compounds. J Org Chem 74:1826–1834
Campeau L, Stuart DR, Leclerc J, Bertrand-Laperle M, Villemure E, Sun H, Lasserre S, Guimond N, Lecavallier M, Fagnou K (2009) Palladium-catalyzed direct arylation of azine and azole N-oxides: reaction development, scope and applications in synthesis. J Am Chem Soc 131:3291–3306
Jafarpour F, Rahiminejadan S, Hazrati H (2010) Triethanolamine-mediated palladium-catalyzed regioselective C-2 direct arylation of free NH-pyrroles. J Org Chem 75:3109–3112
Sattler JJHB, Ruiz-martinez J, Santillan-jimenez E, Weckhuysen BM (2014) Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem Rev 114:10613–10653
Nawaz Z (2015) Light alkane dehydrogenation to light olefin technologies: a comprehensive review. Rev Chem Eng 31:413–436
Vora BV (2012) Development of dehydrogenation catalysts and processes. Top Catal 55:1297–1308
Searles K, Chan KW, Mendes Burak JA, Zemlyanov D, Safonova O, Copéret C (2018) Highly productive propane dehydrogenation catalyst using silica-supported Ga–Pt nanoparticles generated from single-sites highly productive propane dehydrogenation catalyst using silica-supported Ga–Pt nanoparticles generated from single-sites. J Am Chem Soc 140:11674–11679
Al-shaikhali AH, Jedidi A, Anjum DH, Cavallo L, Takanabe K (2017) Kinetics on NiZn bimetallic catalysts for hydrogen evolution via selective dehydrogenation of methylcyclohexane to toluene. ACS Catal 7:1592–1600
Yuan S, Meng L, Wang J (2013) Greatly improved methane dehydrogenation via Ni adsorbed Cu(100) surface. J Phys Chem C 117:14796–14803
Wang S, Zhang D, Ma Y, Zhang H, Gao J, Nie Y, Sun X (2014) Aqueous solution synthesis of Pt–M (M = Fe, Co, Ni) bimetallic nanoparticles and their catalysis for the hydrolytic dehydrogenation of ammonia borane. ACS Appl Mater Interfaces 6:12429–12435
Lei Y, Liu B, Lu J, Lobo-Lapidus RJ, Wu T, Feng H, Xia X, Mane AU, Libera JA, Greeley JP, Miller JT, Elam JW (2012) Synthesis of Pt–Pd core–shell nanostructures by atomic layer deposition: application in propane oxidative dehydrogenation to propylene. Chem Mater 24:3525–3533
Cesar LG, Yang C, Lu Z, Ren Y, Zhang G, Miller JT (2019) Identification of a Pt3Co surface intermetallic alloy in Pt–Co propane dehydrogenation catalysts. ACS Catal 9:5231–5244
Bratko I, Gómez M (2013) Polymetallic complexes linked to a single-frame ligand: cooperative effects in catalysis. Dalton Trans 42:10664–10681
Haak RM, Belmonte MM, Escudero-Adán EC, Benet-Buchholza J, Kleij AW (2010) Olefin metathesis as a tool for multinuclear Co(III) salen catalyst construction: access to cooperative catalysts. Dalton Trans 39:593–602
Kong S, Song K, Liang T, Guo C, Sun W, Redshaw C (2013) Methylene-bridged bimetallic α-diimino nickel(II) complexes: synthesis and high efficiency in ethylene polymerization. Dalton Trans 42:9176–9187
Kuo H, Liu Y, Peng S, Liu S (2012) N,N′-Dialkylation catalyzed by bimetallic iridium complexes containing a saturated bis-N-heterocyclic carbene (NHC) Ligand. Organometallics 31:7248–7255
Sabater S, Mata JA, Peris E (2012) Dual catalysis with an Ir III–AuI heterodimetallic complex: reduction of nitroarenes by transfer hydrogenation using primary alcohols. Chem A Eur J 18:6380–6385
Wang Q, Mikkola S, Lönnberg H (2004) Bimetallic complexes of spiro-azacrown ligands as catalysts of phosphoester and phosphoric anhydride cleavage. Chem Biodivers 1:1316–1326
Xi Z, Zhou Y, Chen W (2008) Efficient Negishi coupling reactions of aryl chlorides catalyzed by binuclear and mononuclear nickel-N-heterocyclic carbene complexes. J Org Chem 73:8497–8501
Yamagiwa N, Matsunaga S, Shibasaki M (2003) Heterobimetallic catalysis in asymmetric 1,4-addition of O-alkylhydroxylamine to enones. J Am Chem Soc 125:16178–16179
Seemeyer K, Schroeder D, Kempf M, Lettau O, Mueller J, Schwarz H (1995) Face selectivity of the C–H bond activation of cyclohexane by the “Bare” first-row transition-metal cations Sc+–Zn+. Organometallics 14:4465–4470
Schlangen M, Schwarz H (2011) Probing elementary steps of nickel-mediated bond activation in gas-phase reactions: ligand- and cluster-size effects. J Catal 284:126–137
MacTaylor RS, Vann WD, Castleman AW (1996) Thermal energy reaction rates of titanium monomer cation. J Phys Chem 100:5329–5333
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09, revision D.01 Gaussian, Inc. Wallingford
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J Chem Phys 132:15410401–15410419
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Legault CY (2009) CYLview (v1.0b561) Universitéde Sherbrooke: http://www.cylview.org
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Yang Y, Hou X, Zhang T, Ma J, Zhang W, Tang S, Sun H, Zhang J (2018) Mechanistic insights into the nickel-catalyzed cross-coupling reaction of benzaldehyde with benzyl alcohol via C–H activation: a theoretical investigation. J Org Chem 83:11905–11916
Ray M, Nakao Y, Sato H, Sakaki S, Watanabe T, Hashimoto H, Tobita H (2010) Experimental and theoretical study of a tungsten dihydride silyl complex: new insight into its bonding nature and fluxional behavior. Organometallics 29:6267–6281
Sakaba H, Oike H, Kawai M, Takami M, Kabuto C, Ray M, Nakao Y, Sato H, Sakaki S (2011) Synthesis, structure, and bonding nature of ethynediyl-bridged bis(silylene) dinuclear complexes of tungsten and molybdenum. Organometallics 30:4515–4531
Ochi N, Nakao Y, Sato H, Sakaki S (2010) 2 + 2 cycloaddition of Alkyne with titanium-imido complex: theoretical study of determining factor of reactivity and regioselectivity. J Phys Chem A 114:659–665
Guan W, Sakaki S, Kurahashi T, Matsubara S (2015) Reasons two nonstrained C–C σ-bonds can be easily cleaved in decyanative [4 + 2] cycloaddition catalyzed by nickel(0)/lewis acid systems. Theoretical insight. ACS Catal 5:1–10
Zeng G, Sakaki S (2012) Unexpected electronic process of H2 activation by a new nickel borane complex: comparison with the usual homolytic and heterolytic activations. Inorg Chem 52:2844–2853
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88:899–926
Dapprich S, Frenking G (1995) Investigation of donor–acceptor interactions: a charge decomposition analysis using fragment molecular orbitals. J Phys Chem 99:9352–9362
Ehlers AW, Dapprich S, Vyboishchikov SF, Frenking G (1996) Structure and bonding of the transition-metal carbonyl complexes M(CO)5L (M = Cr, Mo, W) and M(CO)3L (M = Ni, Pd, Pt; L = CO, SiO, CS, N2, NO+, CN−, NC−, HCCH, CCH2, CH2, CF2, H2)1. Organometallics 15:105–117
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, F., Li, L., Zhang, X. et al. Cooperative effect of hetero-nuclear MnNi+ cation enhancing C–H bond activation of cyclohexane: a theoretical study. Theor Chem Acc 139, 48 (2020). https://doi.org/10.1007/s00214-020-2562-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-2562-7