Abstract
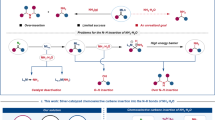
The competing mechanisms of silver-/aminolithium-catalyzed hydrofunctionalization of α,β-unsaturated ester with an amino alcohol have been systematically studied with the DFT methods. Here, the acidity of a weaker nucleophile OH group of an amino alcohol is significantly higher than that of NH2 group, so it is easy to deprotonate by Ag(HMDS)/dppe. However, the generated Ag–O bond is more stable than the corresponding Ag–N bond. Therefore, the OH group shows higher reactivity than the NH2 group in the presence of a Lewis acid/Brønsted base pair catalyst. Then, α,β-unsaturated esters can be inserted into corresponding Ag–O bonds to obtain alkyl silver species. Alkyl silver can be protonated by bis(trimethylsilyl)amines to obtain hydrogen functionalized products and regenerate silver amino active compounds Ag(HMDS)/dppe.



Similar content being viewed by others
References
Michael A, Prakt J (1887) Ueber die addition von natriumacetessig- und natriummalonsäureäthern zu den aethern ungesättigter säuren. Chem 35:349–356. https://doi.org/10.1002/prac.18870350136
Tokoroyama T (2010) Discovery of the Michael reaction. Eur J Org Chem 10:2009–2016. https://doi.org/10.1002/ejoc.200901130
Gardossi LD, Bianchi D, Klibanov AM (1991) Selective acylation of peptides catalyzed by lipases in organic solvents. J Am Chem Soc 113:6328–6329. https://doi.org/10.1021/ja00016a091
Ohshima T, Iwasaki T, Maegawa Y, Yoshiyama A, Mashima K (2008) Enzyme-like chemoselective acylation of alcohols in the presence of Amines Catalyzed by a Tetranuclear Zinc Cluster. J Am Chem Soc 130:2944–2945. https://doi.org/10.1021/ja711349r
Maegawa Y, Ohshima T, Hayashi Y, Agura K, Iwasaki T, Mashima K (2011) Additive effect of N-Heteroaromatics on transesterification catalyzed by Tetranuclear Zinc Cluster. ACS Catal 1:1178–1182. https://doi.org/10.1021/cs200224b
Hatano M, Furuya Y, Shimmura T, Moriyama K, Kamiya S, Maki T, Ishihara K (2011) Ligand-assisted rate acceleration in Lanthanum(III) Isopropoxide catalyzed transesterification of Carboxylic Esters. Org Lett 13:426–429. https://doi.org/10.1021/ol102753n
Lin MH, RajanBabu TV (2000) Metal-catalyzed Acyl transfer reactions of Enol Esters: role of Y5(OiPr)13O and (thd)2Y(OiPr) as transesterification catalysts. Org Lett 2:997–1000. https://doi.org/10.1021/ol0057131
Hayashi Y, Santoro S, Azuma Y, Himo F, Ohshima T, Mashima K (2013) Enzyme-like catalysis via Ternary complex mechanism: Alkoxy-Bridged dinuclear cobalt complex mediates chemoselective O-esterification over N-amidation. J Am Chem Soc 135:6192–6199. https://doi.org/10.1021/ja400367h
Uesugi S, Li Z, Yazaki R, Ohshima T (2014) Chemoselective catalytic conjugate addition of alcohols over amines. Angew Chem Int Ed 53:1611–1615. https://doi.org/10.1002/anie.201309755
Li Z, Yazaki R, Ohshima T (2016) Chemo- and regioselective direct functional group installation through catalytic hydroxy group selective conjugate addition of amino alcohols to α, β-unsaturated sulfonyl compounds. Org Lett 18:3350–3353. https://doi.org/10.1021/acs.orglett.6b01464
Li Z, Tamura M, Yazaki R, Ohshima T (2017) Catalytic chemoselective conjugate addition of amino alcohols to α, β-unsaturated ester: hydroxy group over amino group and conjugate addition over transesterification. Chem Pharm Bull 65:19–21. https://doi.org/10.1248/cpb.c16-00333
Nerush A, Vogt M, Gellrich U, Leitus G, Ben-David Y, Milstein D (2016) Template catalysis by metal-ligand cooperation. c-c bond formation via conjugate addition of non-activated nitriles under mild, base-free conditions catalyzed by a manganese pincer complex. J Am Chem Soc 138:6985–6997. https://doi.org/10.1021/jacs.5b13208
Li W, Werner T (2017) B(C6F5)3-Catalyzed Michael Reactions: Aromatic C-H as Nucleophiles. Org Lett 19:2568–2571. https://doi.org/10.1021/acs.orglett.7b00720
Frayne SH, Murthy RR, Northrop BH (2017) Investigation and demonstration of catalyst/initiator driven selectivity in Thiol-Michael reactions. J Org Chem 82:7946–7956. https://doi.org/10.1021/acs.joc.7b01200
Huang R, Li Z, Yu J, Chen H, Jiang B (2019) H2O regulated chemoselectivity in Oxa- versus Aza-Michael reactions. Org Lett 21:4159–4162. https://doi.org/10.1021/acs.orglett.9b01342
Tang S, Milstein D (2019) Template catalysis by manganese pincer complexes: oxa- and aza-Michael additions to unsaturated nitriles. Chem Sci 10:8990–8994. https://doi.org/10.1039/C9SC03269J
Bai H, Zhang H, Guo Y, Chen H, Wei D, Li S, Zhu Y, Zhang W (2019) Understanding the Z selectivity of the metal-free intermolecular aminoarylation of alkynes: a DFT study. Org Chem Front 6:125–133. https://doi.org/10.1039/C8QO01093E
Bai H, Zhang H, Zhang X, Wang L, Li S, Wei D, Zhu Y, Zhang W (2019) Unravelling the Origins of Hydroboration Chemoselectivity Inversion Using an N, O-Chelated Ir(I) Complex: A Computational Study. J Org Chem 84:6709–6718. https://doi.org/10.1021/acs.joc.9b00329
Wang X, Wang Y, Song J, Wei D (2020) Insights into N-heterocyclic carbene and Lewis acid cooperatively catalyzed oxidative [3 + 3] annulation reactions of α, β-unsaturated aldehyde with 1,3-dicarbonyl compounds. Org Chem Front 7:1113–1121. https://doi.org/10.1039/D0QO00091D
Deng Q, Mu F, Qiao Y, Wei D (2020) A Theoretical Review for the Novel Lewis Base Amine/Imine-Catalyzed Reactions. Org Biomol Chem 18:6781–6800. https://doi.org/10.1039/D0OB01378A
Liu C, Han P, Xie Z, Xu Z, Wei D (2018) Insights into Ag(I)-catalyzed addition reactions of amino alcohols to electron-deficient olefins: competing mechanisms, role of catalyst, and origin of chemoselectivity. RSC Adv 8:40338–40346. https://doi.org/10.1039/C8RA09065C
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. Wallingford, CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian−Type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Dolg M, Wedig U, Stoll H, Preuss H (1987) Energy-adjusted ab initio pseudopotentials for the first row transition elements. J Chem Phys 86:866–872. https://doi.org/10.1063/1.452288
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Fang D-C (2013) THERMO, Beijing Normal University. People’s Republic of China, Beijing
Legault CY, CYLview, 1.0b. Universite′ de Sherbrooke. http://www.cylview.org. 2009.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21703195 and 31601447), the Third Outstanding Young Talent Project of Xuchang University, the Fifth Outstanding Young Teacher Project of Xuchang University, and Training Plan of Young Core Teachers in Universities of Henan Province for the year of 2019 (No. 2019GGJS211).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Han, P., Han, H., Zhang, X. et al. The mechanism studies of catalytic chemoselective conjugate addition of amino alcohols to α,β-unsaturated ester. Theor Chem Acc 140, 6 (2021). https://doi.org/10.1007/s00214-020-02711-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02711-y