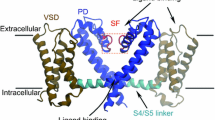
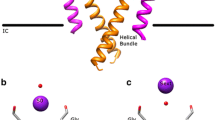
Abstract
Numerous theoretical and experimental studies have attempted to determine the mechanisms of bacterial potassium channel selectivity (KcsA). However, there are still different aspects that remain uncovered. In this paper, we have built a model based on a selective filter (SF) for the KcsA, taking into account its structure and calculating the system interaction energy (cation–water–SF-fragment), using both sodium and potassium as cations. The results tell us which aspects could be responsible for the higher selectivity of the channel. All this reveals that there are two most important aspects: the dehydration of potassium in relation to sodium, and the environment where such dehydration occurs, in the entrance of the SF. Both semi-empirical and ab initio methods are applied to analyse and quantify the change of the interactions that take place when the cation K+ or Na+ crosses the SF.





Similar content being viewed by others
References
MacKinnon R, Cohen SL, Kuo A, Lee A, Chait BT (1998) Structural conservation in prokaryotic and eukaryotic potassium channels. Science 280:106–109
Oakes V, Furini S, Domene C (2020) Effect of anionic lipids on ion permeation through the KcsA K+-channel. Biochim Biophys Acta (BBA)-Biomembr 1862:183406
Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ vonduction and selectivity. Science 280:69–77
Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R (2003) X-ray structure of a voltage-dependent K+ channel. Nature 423:33–41
Clarke OB, Caputo AT, Hill AP, Vandenberg JI, Smith BJ, Gulbis JM (2010) Domain reorientation and rotation of an intracellular assembly regulate conduction in Kir potassium channels. Cell 141:1018–1029
Sun J, MacKinnon R (2020) Structural basis of human kcnq1 modulation and gating. Cell 180(340–347):e9
LeMasurier M, Heginbotham L, Miller C (2001) Kcsa: it’s a potassium channel. J Gen Physiol 118:303–314
Phongphanphanee S, Yoshida N, Oiki S, Hirata F (2014) Distinct configurations of cations and water in the selectivity filter of the KcsA potassium channel probed by 3D-RISM theory. J Mol Liq 200:52–58 Proceedings of the 7th Mini-Symposium on Liquids “Liquid-Liquid Phase Separation and Related Topics on Liquids”
Wang S, Lee SJ, Maksaev G, Fang X, Zuo C, Nichols CG (2019) Potassium channel selectivity filter dynamics revealed by single-molecule fret. Nat Chem Biol 15:377–383
Tilegenova C, Cortes DM, Cuello LG (2017) Hysteresis of KcsA potassium channel’s activation—deactivation gating is caused by structural changes at the channel’s selectivity filter. Proc Natl Acad Sci 114:3234–3239
Eichmann C, Frey L, Maslennikov I, Riek R (2019) Probing ion binding in the selectivity filter of the KcsA potassium channel. J Am Chem Soc 141:7391–7398
He Y, Zhang B, Dong H, Xu P, Cai X, Zhou T, Yu M, Liang J, Zheng X, Tian C (2019) Equilibria between the K+ binding and cation vacancy conformations of potassium channels. Protein Cell 10:533–537
Bliznyuk AA, Rendell AP, Allen TW, Chung SH (2001) The potassium ion channel: comparison of linear scaling semiempirical and molecular mechanics representations of the electrostatic potential. J Phys Chem B 105:12674–12679
Sadhu B, Sundararajan M, Bandyopadhyay T (2015) Selectivity of a singly permeating ion in nonselective NaK channel: combined QM and MD based investigations. J Phys Chem B 119:12783–12797 PMID:26377764
Flood E, Boiteux C, Lev B, Vorobyov I, Allen TW (2019) Atomistic simulations of membrane ion channel conduction, gating, and modulation. Chem Rev 119:7737–7832 PMID:31246417
Kopec W, Rothberg BS, de Groot BL (2019) Molecular mechanism of a potassium channel gating through activation gate-selectivity filter coupling. Nat Commun 10:5366
Callahan KM, Mondou B, Sasseville L, Schwartz JL, D’Avanzo N (2019) The influence of membrane bilayer thickness on KcsA channel activity. Channels 13:424–439
Renart ML, Giudici AM, Diaz-Garcia C, Molina ML, Morales A, González-Ros JM, Poveda JA (2020) Modulation of function, structure and clustering of K+ channels by lipids: lessons learnt from KcsA. Int J Mol Sci 21:2554
Corry B (2018) The naked truth about K+ selectivity. Nat Chem 10:799–800
Kopec W, Köpfer DA, Vickery ON, Bondarenko AS, Jansen TLC, de Groot BL, Zachariae U (2018) Direct knock-on of desolvated ions governs strict ion selectivity in K+ channels. Nat Chem 10:813–820
Li J, Ostmeyer J, Cuello LG, Perozo E, Roux B (2018) Rapid constriction of the selectivity filter underlies C-type inactivation in the KcsA potassium channel. J Gen Physiol 150:1408–1420
Case D, Cerutti D, Cheatham T III, Darden T, Duke R, Giese T, Gohlke H, Goetz A, Greene D, Homeyer N, Izadi S, Kovalenko A, Lee T, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Mermelstein D, Merz K, Monard G, Nguyen H, Omelyan I, Onufriev A, Pan F, Qi R, Roe D, Roitberg A, Sagui C, Simmerling C, Botello-Smith W, Swails J, Walker R, Wang J, Wolf R, Wu X, Xiao L, York D, Kollman P (2017) AMBER. University of California, San Francisco
Stewart J (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J,Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 revision d.01. Gaussian Inc., Wallingford
Schaftenaar G, Noordik J (2000) Molden: a pre- and post-processing program for molecular and electronic structures. J Comput-Aided Mol Des 14:123–134
Jmol: an open-source java viewer for chemical structures in 3D. http://www.jmol.org. Accessed 6 Jan 2021
Schrödinger LLC Pymol the Pymol molecular graphics system, version 1.8, Schrödinger, LLC. https://pymol.org/2. Accessed 6 Jan 2021
Grace: grace is a WYSIWYG 2D plotting tool. http://plasma-gate.weizmann.ac.il/Grace. Accessed 6 Jan 2021
Ohtomo N, Arakawa K (1980) Neutron diffraction study of aqueous ionic solutions. II. Aqueous solutions of sodium chloride and potassium chloride. Bull Chem Soc Jpn 53:1789–1794
Mancinelli R, Botti A, Bruni F, Ricci MA, Soper AK (2007) Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J Phys Chem B 111:13570–13577 PMID:17988114
Azam SS, Hofer TS, Randolf BR, Rode BM (2009) Hydration of sodium(i) and potassium(i) revisited: a comparative QM/MM and QMCF MD simulation study of weakly hydrated ions. J Phys Chem A 113:1827–1834 PMID:19203258
Ferrer J, San-Fabián E (2016) Competition for water between protein (from Haloferax mediterranei) and cations Na+ and K+: a quantum approach to problem. Theor. Chem. Acc. 135:228
Acknowledgements
Financial support by the Spanish “Ministerio de Economia y Competitividad MINECO” (Grants FIS2015-64222-C2-2-P and PID2019-106114GB-I00) and the Universidad de Alicante, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferrer, J., Simó-Cabrera, L. & San-Fabián, E. Energy calculations for potassium vs sodium selectivity on potassium channel: an ab initio study. Theor Chem Acc 140, 16 (2021). https://doi.org/10.1007/s00214-020-02710-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02710-z