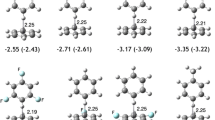
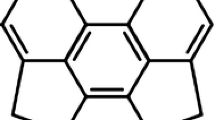
Abstract
A wide variety of methods have been used to study the retro-dimerization reactions of one cis-fused (1) and four new trans-fused benzene dimers (2–5). These methods, which included both single-reference and multi-reference approaches, were benchmarked against multi-reference Møller-Plesset perturbation theory of the second order (MRMP2) based on CASSCF(12,12) wavefunctions. Each of the single-reference approaches was found to be overestimating the activation barrier for retro-dimerization process. Interestingly, N-electron valence perturbation theory (NEVPT2) energies also diverged for these reactions from MRMP2 values. Canonical MP2 barriers were in better agreement with MRMP2 results than the NEVPT2 numbers, with mean unsigned deviations (MUD) of 5.4 kcal/mol and 6.6 kcal/mol, respectively. The behavior of each of the methods is discussed and compared with the application of the same methods to the standard pericyclic reaction set proposed by Houk et al. (J Phys Chem A 107:11445, 2003). The comparison between NEVPT2 and MRMP2 reaction barriers, when applied to the cycloadditions in the Houk set and the synchronous symmetry-allowed retro-dimerization of the benzene dimer 1, showed that these two methods give almost identical results for these reaction types (MUD of 0.5 kcal/mol).




Similar content being viewed by others
References
Woodward RB, Hoffmann R (1969) Angew Chem Int Ed 8:781
Fukui K, Yonezawa T, Shingu H (1952) J Chem Phys 20:722
Diels O, Alder K (1928) Justus Liebigs Ann Chem 460:98
Rogachev AYu, Wen X-D, Hoffmann R (2012) J Am Chem Soc 134:8062
Engelke R, Hay PJ, Kleier DA, Wadt WR (1983) J Chem Phys 79:4367
Engelke R, Hay PJ, Kleier DA, Wadt WR (1984) J Am Chem Soc 106:5439
Schriver GW, Gerson DJ (1990) J Am Chem Soc 112:4723
Wang Y, Li Z, Wang F, Sun C (2009) Phys Chem Chem Phys 11:455
Cometta-Morini C, Baumann H, Oth JFM (1992) J Mol Struct Theochem 277:15 (also Refs. 6 and 7)
Cometta-Morini C, Baumann H, Oth JFM (1992) J Mol Struct Theochem 277:31 (also Refs. 6 and 7)
Engelke R (1986) J Am Chem Soc 108:5799 (also Refs. 7, 8, and 4)
Quenneville J, Germann TC (2009) J Chem Phys 131:024313 (also Refs. 7, 8, and 4)
Zade SS, Zamoshchik N, Reddy RR, Fridman-Marueli G, Shberla D, Bendikov M (2011) J Am Chem Soc 133:10803 (also Refs. 7, 8, and 4)
Bernardi F, Bottoni A, Robb MA, Venturini A (1990) J Am Chem Soc 112:2106
Beno BR, Wilsey S, Houk KN (1999) J Am Chem Soc 121:4816
Koch R, Wentrup C (2004) Org Biomol Chem 2:195
Sakai S (2006) J Phys Chem A 110:6339 (also see Ref. 44)
Dupuis M, Murray C, Davidson ER (1991) J Am Chem Soc 113:9756 (also see Ref. 44)
Wiest O, Black KA, Houk KN (1994) J Am Chem Soc 116:10336 (also see Ref. 44)
Hrovat DA, Morokuma K, Borden WT (1994) J Am Chem Soc 116:1072 (also see Ref. 44)
Koslowski PM, Dupuis M, Davidson ER (1995) J Am Chem Soc 117:774 (also see Ref. 44)
Hrovat DA, Chen J, Houk KN, Borden WT (2000) J Am Chem Soc 122:7656 (also see Ref. 44)
Staroverov VN, Davidson ER (2000) J Am Chem Soc 122:7377 (also see Ref. 44)
Bethke S, Hrovat DA, Borden WT, Gleiter R (2004) J Org Chem 69:3294 (also see Ref. 44)
Blavins JJ, Cooper DL, Karadakov PB (2004) J Phys Chem A 108:194 (also see Ref. 44)
Jiao H, Schleyer PR (1995) Angew Chem Int Ed 34:334 (also see Ref. 44)
Sakai S (2000) Int J Quantum Chem 80:1099 (also see Ref. 44)
Minkin VI, Minyaev RM, Yudilevich IA (1987) Russ J Org Chem 23:1137 (also see Refs. 4 and 44)
Carpenter BK (1992) J Org Chem 57:4645 (also see Refs. 4 and 44)
Lee I, Lee BS, Kim ND, Kim CK (1992) Bull Kor Chem Soc 13:565 (also see Refs. 4 and 44)
Bachrach SM (1993) J Org Chem 58:5414 (also see Refs. 4 and 44)
Kless A, Nendel M, Wilsey S, Houk KN (1999) J Am Chem Soc 121:4524 (also see Refs. 4 and 44)
Mateo A, Sanchez-Andrada P, Lopez-Leonardo C, Alvarez A (2005) J Org Chem 70:7617 (also see Refs. 4 and 44)
Ramig K, Greer EM, Szalda DJ, Razi R, Mahir F, Pokeza N, Wong W, Kaplan B, Lam J, Mannan A, Missak C, Mai D, Subramaniam G, Berkowitz WF, Prasad P, Karimi S, Lo NH, Kudzma LV (2010) Eur J Org Chem 2010:2363 (also see Refs. 4 and 44)
Dolbier WR Jr, Koroniak H, Houk KN, Sheu C (1996) Acc Chem Res 29:471 (see also Refs. 4, 16, and 44)
Jean Y, Volatron F, Nguyen TA (1983) J Mol Struct (Theochem) 10:167 (see also Refs. 4, 16, and 44)
Santiago O, Sole A (1991) J Am Chem Soc 113:8628 (see also Refs. 4, 16, and 44)
Niwayama S, Kallel EA, Sheu C, Houk KN (1996) J Org Chem 61:2517 (see also Refs. 4, 16, and 44)
Niwayama S, Kallel EA, Spellmeyer DC, Sheu C, Houk KN (1996) J Org Chem 61:2813 (see also Refs. 4, 16, and 44)
Faza ON, Lopez CS, Alvarez R, de Lera AR (2004) Org Lett 6:905 (see also Refs. 4, 16, and 44)
Faza O, Lopez CS, Alvarez R, de Lera AR (2004) J Org Chem 69:9002 (see also Refs. 4, 16, and 44)
Mangelinckx S, van Speybroeck V, Vansteenkiste P, Waroquier M, de Kimpe N (2008) J Org Chem 73:5481 (see also Refs. 4, 16, and 44)
Sader CA, Houk KN (2012) J Org Chem 77:4939 (see also Refs. 4, 16, and 44)
Guner V, Khuong KS, Leach AG, Lee PS, Bartberger MD, Houk KN (2003) J Phys Chem A 107:11445
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Perdew JP, Burke K, Ernzerhof M (1997) Phys Rev Lett 78:1396
Adamo C, Barone V (1999) J Chem Phys 110:6158
Erzernhof M, Scuseris G (1999) J Chem Phys 110:5029
Neese F (2012) Wiley Interdiscip Rev Comput Mol Sci 2:73
Dunning TH Jr (1989) J Chem Phys 90:1007
Granovsky AA, Firefly version 8.0, www http://classic.chem.msu.su/gran/firefly/index.html
Neese F, Wennmohs F, Hansen A, Becker U (2009) Chem Phys 356:98
Izsak R, Neese F (2011) J Chem Phys 135:144105
Kendall RA, Früchtl HA (1997) Theor Chem Acc 97:158
Neese F, Schwabe T, Kossmann S, Schirmer B, Grimme S (2009) J Chem Theory Comput 5:3060
Friesner RA (1985) Chem Phys Lett 116:39
Friesner RA (1986) J Chem Phys 85:1462
Friesner RA (1987) J Chem Phys 86:3522
Grimme S (2003) J Chem Phys 118:9095
Kossmann S, Neese F (2010) J Phys Chem A 114:11768 (see also Ref. 54)
Lochan RC, Head-Gordon M (2007) J Chem Phys 126:164101 (see also Refs. 59 and 54)
Hirao K (1992) Chem Phys Lett 190:374
Hirao K (1992) Chem Phys Lett 196:397
Hirao K (1992) Int J Quant Chem S26:517
Hirao K (1993) Chem Phys Lett 201:59
Angeli C, Cimiraglia R, Evangelisti S, Leininger T, Malrieu JP (2001) J Chem Phys 114:10252
Angeli C, Cimiraglia R, Malrieu JP (2001) Chem Phys Lett 350:297
Angeli C, Cimiraglia R (2002) Theor Chem Acc 107:313
Angeli C, Cimiraglia R, Malrieu JP (2002) J Chem Phys 117:9138
Dyall KG (1995) J Chem Phys 102:4909
Schapiro I, Sivalingam K, Neese F (2013) J Chem Theory Comput 9:3567
Granovsky AA (2011) J Chem Phys 134:214113
Nakano H (1993) J Chem Phys 99:7983
Lyakh DI, Musial M, Lotrich VF, Bartlett RJ (2011) Chem Rev 112:182
McNeely J, Rogachev AY (2020) Theor Chem Acc Accepted, Manuscript ID: TCAC-D-20-00070
Bofill JM, Pulay P (1989) J Chem Phys 90:3637
Pulay P, Hamilton TP (1988) J Chem Phys 88:4926
Bone RGA, Pulay P (1992) Int J Quant Chem 45:133
Lee TJ, Taylor PR (1989) Int J Quant Chem S23:199
Cramer CJ, Wloch M, Piecuch P, Puzzarini C, Gagliardi L (1991) J Phys Chem A 2006:110
Liakos DG, Neese F (2011) J Chem Theory Comput 7:1511
Bozkaya U, Sherrill CD (2013) J Chem Phys 138:184103
Kurlancheek W, Head-Gordon M (2009) Mol Phys 107:1223
Gadzhiev OB, Ignatov SK, Krisyuk BE, Maiorov AV, Gangopadhyay S, Masunov AE (2012) J Phys Chem A 116:10420
DeMore WB (1969) J Chem Kinet 1:209
Criegee R (1975) Angew Chem Int Ed 14:745
Acknowledgements
Financial support from Illinois Institute of Technology (start-up funds, A.Y.R) and partial support from the Wanger Institute for Sustainable Energy Research (WISER) Seed Grant (A.Y.R) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McNeely, J., Rogachev, A.Y. New benzene dimers: a benchmark theoretical investigation. Theor Chem Acc 139, 168 (2020). https://doi.org/10.1007/s00214-020-02684-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02684-y