Abstract
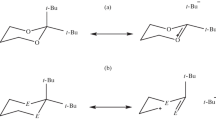
Structures, energies, and electronic properties of anti- and syn-atropisomeric conformers of some chiral imidazolinium salts bearing a substituted aromatic ring have been computed and compared at the B3LYP/6-311+G(d,p) level of density functional theory. Results indicate that the presence of a bulky substituent on the ortho position of the aromatic ring present in these compounds is mainly responsible of the chiral discrimination due to high interconversional energy barriers.







Similar content being viewed by others
References
Rogers RD, Seddon KR (eds) (2003) Ionic liquids as green solvents: progress and prospects. American Chemical Society, Washington, DC
Mévellec V, Leger B, Mauduit M, Roucoux A (2005) Chem Commun 22:2838–2839
Sledź P, Mauduit M, Grela K (2008) Chem Soc Rev 37:2433–2442
Wasserscheid P, Welton T (eds) (2008) Ionic liquids in synthesis, 2nd edn. Wiley, Weinheim
Freemantle M (2009) An introduction to ionic liquids. Royal Society of Chemistry, Cambridge
Aoun B (2010) Liquides ioniques: structure et dynamique. Ph.D. Thesis, University of Orléans
Ohno H (2011) Electrochemical aspects of ionic liquids, 2nd edn. Wiley, Weinheim
Kokorin A (2011) Ionic liquids: applications and perspectives. InTech, India
Sowmiah S, Cheng CI, Chu Y-H (2012) Curr Org Synth 9:74–95
De Los Ríos AP, Fernández FJH (2014) Ionic liquids in separation technology. Elsevier, Amsterdam
Clousier N, Filippi A, Borré E, Guibal E, Crévisy C, Caijo F, Mauduit M, Dez I, Gaumont A-C (2014) ChemSuschem 7:1040–1045
Eftekhari A (2017) Ionic liquid devices. Royal Society of Chemistry, Cambridge
MacFarlane DR, Kar M, Pringle JM (2017) Fundamentals of ionic liquids: from chemistry to applications. Wiley, Weinheim
Welton T (2018) Biophys Rev 10:691–706
Bouchardy L (2016) Elaboration de liquides ioniques (chiraux) réversibles et applications en catalyse organique et en glycochimie. Carbènes N-hétérocycliques chiraux: synthèse et application dans la réaction d’addition conjuguée. Ph.D. Thesis, Paris-Saclay University
Shiflett MB, Scurto AM (2017) ACS Symp Ser 1250:1–13
Zhang Q, Zhang S, Deng Y (2011) Green Chem 13:2619–2637
Neto BAD, Spencer J (2012) J Braz Chem Soc 23:987–1007
Ratti R (2014) Adv Chem 2014:1–16
Hardacre C, Parvulescu V (2014) Catalysis in ionic liquids: from catalyst synthesis to application. The Royal Society of Chemistry, Cambridge
Steinrück H-P, Wasserscheid P (2015) Catal Lett 145:380–397
Romanovsky BV, Tarkhanova IG (2017) Russ Chem Rev 86:444–458
Ozokwelu D, Zhang S, Okafor O, Cheng W, Litombe N (2017) Novel catalytic and separation processes based on ionic liquids. Elsevier, Washington, DC
Xia S-M, Chen K-H, Fu H-C, He L-N (2018) Front Chem 6:462–469
Karimi B, Tavakolian M, Akbari M, Mansouri F (2018) Chem Cat Chem 10:3173–3205
Lozano P (2018) Sustainable catalysis in ionic liquids. CRC Press, Boca Raton
Vidal L, Riekkola ML, Canals A (2012) Anal Chim Acta 715:19–41
Patel DD, Lee JM (2012) Chem Rec 12:329–355
Lei Z, Dai C, Zhu J, Chen B (2014) AIChE J 60:3312–3329
Han D, Row KH (2010) Molecules 15:2405–2426
Rodríguez H (2016) Ionic liquids for better separation processes. Springer, Berlin
Cowan MG, Gin DL, Noble RD (2016) Acc Chem Res 49:724–732
Paul A, Mandal PK, Samanta A (2005) J Phys Chem B 109:9148–9153
Vioux A, Viau L, Volland S, Le Bideau J (2010) CR Chim 13:242–255
Castro Ruiz CA (2012) Évaluation de nouveaux électrolytes à base de liquides ioniques protiques en supercapacités asymétriques de type MnO2/carbone. Ph.D. Thesis, University of Montreal
Behera K, Pandey S, Kadyan A, Pandey S (2015) Sensors 15:30487–30503
Shahvelayati AS, Sabbaghan M, Bashtani SE (2015) Int J Nanosci Nanotechnol 11:123–131
Muginova SV, Myasnikova DA, Kazarian SG, Shekhovtsova TN (2017) Anal Sci 33:261–274
Renuga V, Manikandan A, Mohan CN, Meenatchi B, Ganga B (2017) J Mol Liq 244:65–76
Fernández CDR, Arosa Y, Algnamat BS, Lago E-L, Varela LM, De la Fuente R (2019) Front Opt 10:1364–1397
Gelinas B (2017) Liquides ioniques électroactifs dans la composition d’électrolytes avancés pour des applications en énergie. Doctoral Thesis, University of Montreal
Matsumoto K, Hwang J, Kaushik S, Chen C-Y, Hagiwara R (2019) Energy Environ Sci 12:3247–3287
Gao X, Wu F, Mariani A, Passerini S (2019) ChemSuschem 12:4185–4193
Angell M, Zhu G, Lin M-C, Rong Y, Dai H (2019) Adv Funct Mater 30:1928
Kamijo T, Arafune H, Morinaga T, Honma S, Sato T, Hino M, Mizukami M, Kurihara K (2015) Langmuir 31:13265–13270
Voeltzel N (2016) Molecular simulation of an ionic liquid as lubricant: from bulk rheology to nanoconfinement. Doctoral Thesis, National Institute of Applied Sciences, Lyon
Rohlmann P, Munavirov B, Furó I, Antzutkin O, Rutland MW, Glavatskih S (2019) Front Chem 7:98–106
Avilés M-D, Pamies R, Sanes J, Carrión F-J, Bermúdez M-D (2019) Coatings 9:710–720
Smiglak M, Pringle JM, Lu X, Han L, Zhang S, Gao H, MacFarlane DR, Rogers RD (2014) Chem Commun 50:9228–9250
Domingos S, Andre V, Quaresma S, Martins IC, Minas da Piedade MF, Duarte MT (2015) J Pharm Pharmacol 67:830–846
Dias AR, Costa-Rodrigues J, Fernandes MH, Ferraz R, Prudêncio C (2016) ChemMedChem 12:11–18
Giszter R, Fryder M, Marcinkowska K, Sznajdrowska A (2016) J Braz Chem Soc 27:1774–1781
Egorova KS, Gordeev EG, Ananikov VP (2017) Chem Rev 117:7132–7189
Miskiewicz A, Ceranowicz P, Szymczak M, Bartu’s K, Kowalczyk P (2018) Int J Mol Sci 19:2779–2803
Shah MUH, Sivapragasam M, Moniruzzaman M, Talukder MMR, Yusup SB, Goto M (2018) J Mol Liq 266:568–576
Wang Z, Zhang J, Lu B, Li Y, Liang Y, Yuan J, Zhao M, Wang B, Mai C, Zhang J (2019) J Mol Liq 296:111822
Gomes JM, Silva SS, Reis RL (2019) Chem Soc Rev 48:4317–4335
Tigineh GT, Abebe A (2019) Bioinorg Chem Appl 10:1155–1163
Jovanović-Šanta S, Kojić V, Atlagić K, Tot A, Vraneš M, Gadžurić S, Karaman M (2019) J Serb Chem Soc 84:1–13
Tang J, Song H, Feng X, Yohannes A, Yao S (2019) Curr Med Chem 26:5947–5967
Schrekker HS, Donato RK, Fuentefria AM, Bergamo V, Oliveira LF, Machado MM (2013) MedChemComm 4:1457–1460
Riduan SN, Zhang Y (2013) Chem Soc Rev 42:9055–9070
Odžak R, Skočibušić M, Maravić A (2013) Bioorg Med Chem 21:7499–7506
Elshaarawy RF, Janiak C (2014) Eur J Med Chem 75:31–42
Reinhardt A, Horn M, Schmauck JP, Bröhl A, Giernoth R, Oelkrug C, Schubert A, Neundorf I (2014) Bioconjug Chem 25:2166–2174
Gravel J, Schmitzer AR (2017) Org Biomol Chem 15:1051–1071
Yuan Y, Zhang Y (2017) ChemMedChem 12:835–840
Ocakoglu K, Tasli H, Limoncu MH, Lambrecht FY (2018) Trends Cancer Res Chemother. https://doi.org/10.15761/tcrc.1000103
Hryniewicka A, Malinowska M, Hauschild T, Pieczul K, Morzycki JW (2019) J Steroid Biochem Mol Biol 189:65–72
Deng G, Zhou B, Wang J, Chen Z, Gong L, Gong Y, Wu D, Li Y, Zhang H, Yang X (2019) Eur J Med Chem 168:232–252
Bal S, Kaya R, Gök Y, Taslimi P, Aktaş A, Karaman M, Gülçin İ (2020) Bioorg Chem 94:103468
Deetlefs M, Fanselow M, Seddon KR (2016) RSC Adv 6:4280–4288
Heckenbach ME, Romero FN, Green MD, Halden RU (2016) Chemosphere 150:266–274
Shamshina JL, Kelley SP, Gurau G, Rogers RD (2015) Nature 528:188–189
Clavier H, Coutable L, Toupet L, Guillemin J-C, Mauduit M (2005) J Organomet Chem 690:5237–5254
Clavier H, Coutable L, Guillemin J-C, Mauduit M (2005) Tetrahedron Asymmetry 16:921–924
Martin D, Kehrli S, Augustin M, Clavier H, Mauduit M, Alexakis A (2006) J Am Chem Soc 128:8416–8417
Handy ST (2006) J Org Chem 71:4659–4662
Clavier H, Nolan SP, Mauduit M (2008) Organometallics 27:2287–2292
Winkel A, Wilhelm R (2009) Tetrahedron Asymmetry 20:2344–2350
Borre E, Caijo F, Crévisy C, Mauduit M (2009) Chim Oggi 27:20–24
McGarrigle EM, Fritz SP, Favereau L, Yar M, Aggarwal VK (2011) Org Lett 13:3060–3063
Yang L, Sun R, Zhang L, Li Y, Cao C, Pang G, Shi Y (2011) J Chem Res 35:608–610
Benhamou L, Chardon E, Lavigne G, Laponnaz SB, Cesar V (2011) Chem Rev 111:2705–2733
Germain N, Magrez M, Kehrli S, Mauduit M, Alexakis A (2012) Eur J Org Chem 2012:5301–5306
Thomasset A (2013) Synthèse de carbènes N-hétérocycliques chiraux et applications en catalyse asymétrique. Ph.D. Thesis, University of Paris Sud
Guerria M, Sekhri L, Olivier C, Jean-Luc P (2014) Compd Orient J Chem 30:427–434
Jahier-Diallo C, Morin MST, Queval P, Rouen M, Artur I, Querard P, Toupet L, Crévisy C, Baslé O, Mauduit M (2015) Chem Eur J 21:993–997
Hellou N, Jahier-Diallo C, Baslé O, Srebro-Hooper M, Toupet L, Roisnel T, Caytan E, Roussel C, Vanthuyne N, Autschbach J, Mauduit M, Crassous J (2016) Chem Commun 52:9243–9246
Tarrieu R, Dumas A, Thongpaen J, Vives T, Roisnel T, Dorcet V, Crévisy C, Baslé O, Mauduit M (2017) J Org Chem 82:1880–1887
Curbet I, Morvan J, Rouen SC, Roisnel T, Crévisy C, Mauduit M (2019) Arkivoc 4:102–112
Rix D, Labat S, Toupet L, Crévisy C, Mauduit M (2009) Eur J Inorg Chem 2009:1989–1999
Clavier H, Boulanger L, Audic N, Toupet L, Mauduit M, Guillemin J-C (2004) Chem Commun 2004:1224–1225
Kong L, Morvan J, Pichon D, Jean M, Albalat M, Vives T, Colombel-Rouen S, Giorgi M, Dorcet V, Roisnel T, Crévisy C, Nuel D, Nava P, Humbel S, Vanthuyne N, Mauduit M, Clavier H (2020) J Am Chem Soc 142:93–98
Hohenberg P, Kohn W (1964) Phys Rev B 136:864–871
Kohn W, Sham LJ (1965) Phys Rev A 140:1133–1138
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. University Press UK, Oxford
Dreizler RM, Gross EKV (1990) Density functional theory: an approach to the manybody problem. Springer, Berlin
Koch W, Holthausen MC (2000) A chemist’s guide to density functional theory. Wiley, Weinheim
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian16, Revision B.01, Gaussian Inc., Wallingford, CT. http://www.gaussian.com
Mardirossiana N, Head-Gordon N (2017) Mol Phys 115:2315–2372
Becke AD (1993) J Chem Phys 98:1372–1377
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32:1456–1465
NBO 5.0., Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) Theoretical Chemistry Institute. University of Wisconsin, Madison, WI. http://www.chem.wisc.edu/~nbo5
Reed AE, Weinhold F (1983) J Chem Phys 78:4066–4073
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Gorelsky SI (2009) AOMix program, program for molecular orbital analysis, Rev 6.42. University of Ottawa: Ottawa. http://www.sg-chem.net/aomix
Gorelsky SI, Lever ABP (2001) J Organomet Chem 635:187–196
Flükiger P, Lüthi HP, Portmann S, Weber J (2000-2002) Molekel 4.3. Swiss Center for Scientific Computing: Manno. http://www.cscs.ch/molekel
Wiberg KB (1966) Tetrahedron 24:1083–1096
Mayer I (2006) J Comput Chem 28:204–221
Acknowledgements
AL acknowledges a doctoral fellowship and travel grants from the PROFAS French-Algerian Program and the Algerian-French Program Tassili-07MDU700, respectively. We thank Dr Marc Mauduit (University of Rennes) for providing experimental data.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ladjarafi, A., Meghezzi, H. & Halet, JF. A theoretical investigation on conformers of imidazolinium salts. Theor Chem Acc 139, 165 (2020). https://doi.org/10.1007/s00214-020-02677-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02677-x