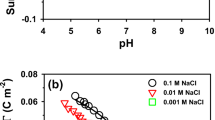
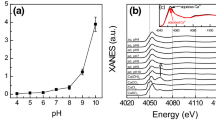
Abstract
The sorption mechanism between 4-chloro-2-methylphenoxyacetic acid (MCPA) herbicide and the dominating (110) surface of the mineral goethite was studied by molecular modeling of the full set of possible surface complexes using density functional theory with periodic boundary conditions for the structural surface models. The most stable arrangements of the MCPA species were predicted taking into account the type and topology of the surface OH groups, protonation states (pH effect), the structure of carboxyl/carboxylate group of MCPA, and the binding type (outer- or inner-sphere complexes). Acid–base properties of MCPA and the goethite surface OH groups led to creation of several pH ranges (3–4, 4–9, 9) for combining neutral/deprotonated MCPA with neutral/protonated goethite surface. The predicted strongest adsorption (physisorption) for the complexes in the pH 4–9 range was followed by largest solvent destabilization of the outer-sphere complexes due to the high solvent energy of the MCPA and surface hydration of the hydroxylated goethite surface. In line with experimental data, the adsorption of MCPA should increase with decreasing pH owing to the presence of neutral MCPA molecule (pKa ~ 3) and its lower solvation energy that can produce more stable complexes in solution than that of anionic MCPA in pH 4–9 range. The formation of the inner-sphere chemisorbed surface complex contributes significantly to the overall adsorption of MCPA at acidic pH range. In the chemisorbed inner-sphere complexes, monodentate binding was revealed through the formation of a Fe–O–C bridge.







Similar content being viewed by others
References
Walker M, Lawrence H (1992) EPA's pesticide fact sheet database. Lewis Publishers Inc., Chelsea
Haberhauer G, Pfeiffer L, Gerzabek MH (2000) Influence of molecular structure on sorption of phenoxyalkanoic herbicides on soil and its particle size fractions. J Agric Food Chem 48(8):3722–3727. https://doi.org/10.1021/jf9912856
Haberhauer G, Pfeiffer L, Gerzabek MH, Kirchmann H, Aquino AJA, Tunega D, Lischka H (2001) Response of sorption processes of MCPA to the amount and origin of organic matter in a long-term field experiment. Eur J Soil Sci 52(2):279–286. https://doi.org/10.1046/j.1365-2389.2001.00382.x
Socias-Viciana MH, Fernandez-Perez M, Villafranca-Sanchez R, Gonzalez-Pradas E, Flores-Cespedes F (1999) Sorption and leaching of atrazine and MCPA in natural and peat-amended calcareous soils from Spain. J Agric Food Chem 47(3):1236–1241. https://doi.org/10.1021/jf980799m
Clausen L, Fabricius I (2001) Atrazine, isoproturon, mecoprop, 2,4-D, and bentazone adsorption onto iron oxides. J Environ Qual 30(3):858–869. https://doi.org/10.2134/jeq2001.303858x
Inacio J, Taviot-Gueho C, Forano C, Besse JP (2001) Adsorption of MCPA pesticide by MgAl-layered double hydroxides. Appl Clay Sci 18(5–6):255–264. https://doi.org/10.1016/s0169-1317(01)00029-1
Vasudevan D, Cooper EM, Van Exem OL (2002) Sorption-desorption of lonogenic compounds at the mineral-water interface: study of metal oxide-rich soils and pure-phase minerals. Environ Sci Technol 36(3):501–511. https://doi.org/10.1021/Es0109390
Thorstensen CW, Lode O (2001) Laboratory degradation studies of bentazone, dichlorprop, MCPA, and propiconazole in Norwegian soils. J Environ Qual 30(3):947–953. https://doi.org/10.2134/jeq2001.303947x
Pignatello JJ, Xing BS (1996) Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol 30(1):1–11. https://doi.org/10.1021/Es940683g
Benoit P, Barriuso E, Calvet R (1998) Biosorption characterization of herbicides, 2,4-D and atrazine, and two chlorophenols on fungal mycelium. Chemosphere 37(7):1271–1282. https://doi.org/10.1016/S0045-6535(98)00125-8
Bolan NS, Baskaran S (1996) Biodegradation of 2,4-D herbicide as affected by its adsorption–desorption behaviour and microbial activity of soils. Aust J Soil Res 34(6):1041–1053. https://doi.org/10.1071/Sr9961041
DePaolis F, Kukkonen J (1997) Binding of organic pollutants to humic and fulvic acids: Influence of pH and the structure of humic material. Chemosphere 34(8):1693–1704. https://doi.org/10.1016/S0045-6535(97)00026-X
Sannino F, Violante A, Gianfreda L (1997) Adsorption-desorption of 2,4-D by hydroxy aluminium montmorillonite complexes. Pestic Sci 51(4):429–435. https://doi.org/10.1002/(sici)1096-9063(199712)51:4<429:aid-ps619>3.0.co;2-j
Susarla S, Bhaskar GV, Bhamidimarri SMR (1997) Competitive adsorption-desorption kinetics of phenoxyacetic acids and a chlorophenol in volcanic soil. Environ Technol 18(9):937–943. https://doi.org/10.1080/09593331808616613
Celis R, Hermosin MC, Cox L, Cornejo J (1999) Sorption of 2,4-dichlorophenoxyacetic acid by model particles simulating naturally occurring soil colloids. Environ Sci Technol 33(8):1200–1206. https://doi.org/10.1021/es980659t
Cox L, Celis R, Hermosin MC, Cornejo J (2000) Natural soil colloids to retard simazine and 2,4-d leaching in soil. J Agric Food Chem 48(1):93–99. https://doi.org/10.1021/Jf990585k
Spadotto CA, Hornsby AG (2003) Soil sorption of acidic pesticides: modeling pH effects. J Environ Qual 32(3):949–956. https://doi.org/10.2134/jeq2003.9490
Jensen PH, Hansen HCB, Rasmussen J, Jacobsen OS (2004) Sorption-controlled degradation kinetics of MCPA in soil. Environ Sci Technol 38(24):6662–6668. https://doi.org/10.1021/Es0494095
Aquino AJA, Tunega D, Haberhauer G, Gerzabek MH, Lischka H (2007) Quantum chemical adsorption studies on the (110) surface of the mineral goethite. J Phys Chem C 111(2):877–885. https://doi.org/10.1021/Jp0649192
Tunega D, Gerzabek MH, Haberhauer G, Totsche KU, Lischka H (2009) Model study on sorption of polycyclic aromatic hydrocarbons to goethite. J Colloid Interface Sci 330(1):244–249. https://doi.org/10.1016/j.jcis.2008.10.056
Tunega D, Haberhauer G, Gerzabek MH, Lischka H (2004) Sorption of phenoxyacetic acid herbicides on the kaolinite mineral surface—an ab initio molecular dynamics simulation. Soil Sci 169(1):44–54. https://doi.org/10.1097/01.ss.0000112015.97541.f3
Tunega D, Gerzabek MH, Haberhauer G, Lischka H (2007) Formation of 2,4-D complexes on montmorillonites—an ab initio molecular dynamics study. Eur J Soil Sci 58(3):680–691. https://doi.org/10.1111/j.1365-2389.2006.00853
Kersten M, Tunega D, Georgieva I, Vlasova N, Branscheid R (2014) Adsorption of the herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) by goethite. Environ Sci Technol 48(20):11803–11810. https://doi.org/10.1021/Es502444c
Werner D, Garratt JA, Pigott G (2013) Sorption of 2,4-D and other phenoxy herbicides to soil, organic matter, and minerals. J Soil Sediment 13(1):129–139. https://doi.org/10.1007/s11368-012-0589-7
Pronk GJ, Heister K, Kogel-Knabner I (2011) Iron oxides as major available interface component in loamy arable topsoils. Soil Sci Soc Am J 75(6):2158–2168. https://doi.org/10.2136/sssaj2010.0455
Iglesias A, Lopez R, Gondar D, Antelo J, Fiol S, Arce F (2010) Adsorption of MCPA on goethite and humic acid-coated goethite. Chemosphere 78(11):1403–1408. https://doi.org/10.1016/j.chemosphere.2009.12.063
Angove MJ, Fernandes MB, Ikhsan J (2002) The sorption of anthracene onto goethite and kaolinite in the presence of some benzene carboxylic acids. J Colloid Interface Sci 247(2):282–289. https://doi.org/10.1006/jcis.2001.8133
Muller S, Totsche KU, Kogel-Knabner I (2007) Sorption of polycyclic aromatic hydrocarbons to mineral surfaces. Eur J Soil Sci 58(4):918–931. https://doi.org/10.1111/j.1365-2389.2007.00930.x
Weigand H, Totsche KU (1998) Flow and reactivity effects on dissolved organic matter transport in soil columns. Soil Sci Soc Am J 62(5):1268–1274. https://doi.org/10.2136/sssaj1998.03615995006200050017x
Gaboriaud F, Ehrhardt J (2003) Effects of different crystal faces on the surface charge of colloidal goethite (alpha-FeOOH) particles: an experimental and modeling study. Geochim Cosmochim Ac 67(5):967–983. https://doi.org/10.1016/S0016-7037(02)00988-2
Kavanagh BV, Posner AM, Quirk JP (1977) Adsorption of phenoxyacetic acid herbicides on goethite. J Colloid Interface Sci 61(3):545–553. https://doi.org/10.1016/0021-9797(77)90472-6
Iglesias A, Lopez R, Gondar D, Antelo J, Fiol S, Arce F (2009) Effect of pH and ionic strength on the binding of paraquat and MCPA by soil fulvic and humic acids. Chemosphere 76(1):107–113. https://doi.org/10.1016/j.chemosphere.2009.02.012
Boily JF, Persson P, Sjoberg S (2000) Benzenecarboxylate surface complexation at the goethite (alpha-FeOOH)/water interface: II. Linking IR spectroscopic observations to mechanistic surface complexation models for phthalate, trimellitate, and pyromellitate. Geochim Cosmochim Acta 64(20):3453–3470. https://doi.org/10.1016/S0016-7037(00)00453-1
Aquino AJA, Tunega D, Haberhauer G, Gerzabek MH, Lischka H (2008) Acid–base properties of a goethite surface model: a theoretical view. Geochim Cosmochim Acta 72(15):3587–3602. https://doi.org/10.1016/j.gca.2008.04.037
Kresse G, Furthmuller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186. https://doi.org/10.1103/physrevb.54.11169
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868. https://doi.org/10.1103/physrevlett.77.3865
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59(3):1758–1775. https://doi.org/10.1103/PhysRevB.59.1758
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys Rev B 57(3):1505–1509. https://doi.org/10.1103/PhysRevB.57.1505
Otte K, Pentcheva R, Schmahl WW, Rustad JR (2009) Pressure-induced structural and electronic transitions in FeOOH from first principles. Phys Rev B. https://doi.org/10.1103/Physrevb.80.205116
Tunega D (2012) Theoretical study of properties of goethite (alpha-FeOOH) at ambient and high-pressure conditions. J Phys Chem C 116(11):6703–6713. https://doi.org/10.1021/Jp2091297
Hazemann JL, Berar JF, Manceau A (1991) Rietveld studies of the aluminum-iron substitution in synthetic goethite. Mater Sci Forum 79:821–826. https://doi.org/10.4028/www.scientific.net/MSF.79-82.821
Bucko T, Hafner J, Angyan JG (2005) Geometry optimization of periodic systems using internal coordinates. J Chem Phys. https://doi.org/10.1063/1.1864932
Baker J, Kessi A, Delley B (1996) The generation and use of delocalized internal coordinates in geometry optimization. J Chem Phys 105(1):192–212. https://doi.org/10.1063/1.471864
Manz TA, Limas NG (2016) Introducing DDEC6 atomic population analysis: part 1. Charge partitioning theory and methodology. RSC Adv 6(53):47771–47801. https://doi.org/10.1039/C6RA04656H
Manz T, Limas NG (2017) Chargemol program for performing DDEC analysis. https://ddec.sourceforge.net/. Accessed Aug 2017.
Manz TA (2017) Introducing DDEC6 atomic population analysis: part 3. Comprehensive method to compute bond orders. RSC Adv 7(72):45552–45581. https://doi.org/10.1039/c7ra07400j
Manz TA, Yang B (2016) Selective oxidation passing through eta(3)-ozone intermediates: applications to direct propene epoxidation using molecular oxygen oxidant (vol 4, pg 27755, 2014). RSC Adv 6(110):108153. https://doi.org/10.1039/C4RA03729D
Desiraju GR, Steiner T (2001) The weak hydrogen bond in structural chemistry and biology. OUP, Chichester
Ahlrichs R, Bar M, Haser M, Horn H, Kolmel C (1989) Electronic-structure calculations on workstation computers—the program system turbomole. Chem Phys Lett 162(3):165–169. https://doi.org/10.1016/0009-2614(89)85118-8
Schafer A, Huber C, Ahlrichs R (1994) Fully optimized contracted gaussian-basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100(8):5829–5835. https://doi.org/10.1063/1.467146
Klamt A, Schuurmann G (1993) Cosmo—a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc Perk T 2(5):799–805. https://doi.org/10.1039/P29930000799
Acknowledgements
We are grateful for the financial support the German Research Foundation (No. GE 1676/1) of priority research program SPP 1315. I.G thanks for the financial support the Bulgarian National Science Fund of Bulgarian Ministry of Education and Science, Grant DH09/9/2016. The authors also acknowledge the technical support and computer time at the Vienna Scientific Computing (VSC) cluster.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Festschrift in honor of Prof. Fernando R. Ornellas” Guest Edited by Adélia Justino Aguiar Aquino, Antonio Gustavo Sampaio de Oliveira Filho and Francisco Bolivar Correto Machado.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Georgieva, I., Kersten, M. & Tunega, D. Molecular modeling of MCPA herbicide adsorption by goethite (110) surface in dependence of pH. Theor Chem Acc 139, 132 (2020). https://doi.org/10.1007/s00214-020-02646-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02646-4