Abstract
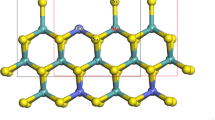
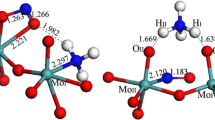
In this paper, the stable structure of Mo-edge of 2H-MoS2 in water and the H2 evolution mechanism at Mo-edge in 2H-Mo7S17 cluster were investigated by the B3LYP method of the density functional theory. The calculations suggested that the stable structure of the Mo-edge in gas and water was different. The Mo-edge with the upright S bonded by one Mo atom was more stable in water while the S atom of Mo-edge was bonded by two Mo atoms to generate a stable Mo-edge in gas. The adsorption energy of H on S was higher than that on Mo at Mo-edge; as a result, the hydrogen evolution reactions on S and Mo were limited by the Heyrovsky and Volmer step, respectively. In hydrogen evolution reaction, the Volmer reaction occurs on S to produce Mo7S17HS, leading to the aggregation of electron on Mo and thus decreasing the barriers for H2 evolution reaction on Mo. Subsequently, the Mo in Mo7S17HS severing as active sites efficiently catalyzed hydrogen evolution reaction through the Volmer–Heyrovsky mechanism, in which the Volmer reaction was identified as the rate-determining step with a potential barrier of 17.9 kcal/mol, being close to the experimental value of 19.9 kcal/mol.




Similar content being viewed by others
References
Walter MG, Warren EL, McKone JR et al (2010) Solar water splitting cells. Chem Rev 110:6446–6473
Gratzal M (2001) Photoelectrochemical cells. Nature 414:338–344
Huang C, Wang X, Wang D et al (2019) Atomic pillar effect in PdxNbS2 to boost basal plane activity for stable hydrogen evolution. Chem Mater 31:4726–4731
Kong C, Min S, Lu G (2014) Robust Pt-Sn alloy decorated graphene nanohybrid cocatalyst for photocatalytic hydrogen evolution. Chem Commun 50:9281–9283
Zhang G, Lan ZA, Lin L et al (2016) Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem Sci 7:3062–3066
He J, Wang J, Chen Y et al (2014) A dye-sensitized Pt@UiO-66(Zr) metal-organic framework for visible-light photocatalytic hydrogen production. Chem Commun 50:7063–7066
Wang N, Wang J, Hu J et al (2018) Design of palladium-doped g-C3N4 for enhanced photocatalytic activity toward hydrogen evolution reaction. ACS Appl Energy Mater 1:2866–2873
Ganguly P, Harb M, Cao Z et al (2019) 2D nanomaterials for photocatalytic hydrogen production. ACS Energy Lett 47:1687–1709
Liu T, Liu X, Bhattacharya S et al (2019) Plasma-induced fabrication and straining of MoS2 films for the hydrogen evolution reaction. ACS Appl Energy Mater 2:5162–5170
Zong X, Na Y, Wen F et al (2009) Visible light driven H2 production in molecular systems employing colloidal MoS2 nanoparticles as catalyst. Chem Commun 14:4536–4538
Hinnemann B, Moses PG, Bonde J et al (2005) Biominmeic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J Am Chem Soc 127:5308–5309
Merki D, Hu X (2011) Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ Sci 4:3878–3888
Benck JD, Hellstern TR, Kibsgaard J et al (2014) Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal 4:3957–3971
Vesborg PCK, Seger B, Chorkendorff I (2015) Recent development in hydrogen evolution reaction catalysts and their practical implementation. J Phys Chem Lett 6:951–957
Wang Y, Deng J, Wang X et al (2018) Small stoichiometric (MoS2)n clusters with the 1T phase. Phys Chem Chem Phys 20:6365–6373
Jaramillo TF, Jorgensen KP, Bonde J et al (2007) Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalyst. Science 317:100–102
Huang Y, Nielsen RJ, Goddard WA et al (2015) The reaction mechanism with free energy barrier for electrochemical dihydrogen evolution on MoS2. J Am Chem Soc 137:6692–6698
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Lu T (2017) Multiwfn, version 3.4.1, a multifunctional wavefunction analyzer. http://multiwfn.codeplex.com
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comp Chem 11:361–373
Ganji MD, Hosseini-khah SM, Amini-tabar Z (2015) Theoretical insight into hydrogen adsorption onto graphene: a first-principles B3LYP-D3 study. Phys Chem Chem Phys 17:2504–2511
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision E.01, Gaussian Inc., Wallingford CT
Shama S, Groves MN, Fennell J et al (2014) Carboxyl group enhanced CO tolerant GO supported Pt catalysts: DFT and electrochemical analysis. Chem Mater 26:6142–6151
Fernando A, Weerawardene KLDM, Karimova NV et al (2015) Quantum mechanical studies of large metal, metal oxide, and metal chalcogenide nanoparticles and clusters. Chem Rev 115:6112–6216
Kong C, Han Y, Hou L et al (2017) Theoretical research on the H2 generation mechanism on Pt6, Pt5Sn5 and Pt3Sn6 clusters by density functional theory. Int J Hydrog Energy 42:16157–16169
Raybaud P, Hafner J, Kresse G et al (2000) Ab initio study of the H2–H2S/MoS2 gas-solid interface: the nature of the catalytically active sites. J Catal 189:129–146
Nørskov JK, Bligaard T, Logadottir A et al (2005) Trends in the exchange current for hydrogen evolution. J Electronchem Soc 152:J23–J26
Deng J, Ren P, Deng D et al (2015) Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew Chem Int Ed 54:2100–2104
Gao M, Liang J, Zheng Y et al (2015) An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat Commun 6:5982
Li H, Tsai C, Koh AL et al (2016) Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat Mater 15:48–53
Tang Q, Jiang D (2016) Mechanism of hydrogen evolution reaction on 1T-MoS2 from first principles. ACS Catal 6:4953–4961
Kim KY, Lee J, Kang S et al (2018) Role of hyper-reduced states in hydrogen evolution reaction at sulfur vacancy in MoS2. ACS Catal 8:4508–4515
Kong D, Wang H, Cha JJ et al (2013) Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett 13:1341–1347
Lukowski MA, Daniel AS, Meng F et al (2013) Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J Am Chem Soc 135:10274–10277
Zhu J, Wang ZC, Dai H et al (2019) Boundary activated hydrogen evolution reaction on monolayer MoS2. Nat Commun 10:1348
Acknowledgements
This study was supported by the Doctor Foundation of Long-dong University (XYBY1904), the Key Discipline of Gansu Province, the Innovation Team Project of Gansu University (2018C-22) and the Hexi University Principle Fund of Scientific Innovation and Application (No. XZZD2018004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, YX., Kong, C. & Yan, PJ. Mechanism of hydrogen generation on stable Mo-edge of 2H-MoS2 in water from density functional theory. Theor Chem Acc 139, 98 (2020). https://doi.org/10.1007/s00214-020-02614-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02614-y