Abstract
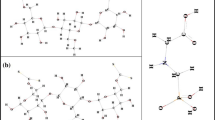
Cellulose derivatives have been synthesized and used in various applications with emphasis on possible application in environmental remediation. In this context, the present work theoretically studied the adsorption of Cr3+, Cu2+ and Cd2+ metal ions in the carboxymethylcellulose (CMC) and cellulose xanthate (CX) matrices, both derived from cellulose. From the calculations, it was possible to obtain map of electrostatic potential, frontier molecular orbitals, reactivity indices, and with these, analyses infer that the cations would interact with the CMC and CX oxygen and also with the CX sulfo group. After complexation, the results showed that the CX and CMC matrices studied have potential to be used to remove toxic metals and presented chemical adsorption and the processes occur spontaneously (ΔG < 0). The topological analysis of quantum theory of atoms in molecules allowed to characterize the nature of the interaction, in which the interactions in position “b” of CMC–Cu2+, CMC–Cd2+ and position “a” of CMC–Cd2+ and “a” in the complexes XC–Cu2+, XC–Cd2+ and “b” in the XC–Cr3+ site (1) presented electrostatic characteristics, and the other interactions were partially covalent. The results found when compared to the study of cellulose and cellulose acetate adsorption with the same metal ions showed that the theoretical data provide insights for a possible experimental approach and use of this matrices.


Similar content being viewed by others
References
de Oliveira Barud HG, da Silva RR, da Silva Barud H et al (2016) A multipurpose natural and renewable polymer in medical applications: bacterial cellulose. Carbohydr Polym 153:406–420. https://doi.org/10.1016/j.carbpol.2016.07.059
Lebedeva NSh, Guseinov SS, Yurina ES et al (2019) Thermochemical research of chitosan complexes with sulfonated metallophthalocyanines. Int J Biol Macromol 137:1153–1160. https://doi.org/10.1016/j.ijbiomac.2019.07.051
Pereira AKDS, Reis DT, Barbosa KM et al (2020) Antibacterial effects and ibuprofen release potential using chitosan microspheres loaded with silver nanoparticles. Carbohydr Res 488:107891. https://doi.org/10.1016/j.carres.2019.107891
Van de Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polym Test 21:433–442. https://doi.org/10.1016/S0142-9418(01)00107-6
Reis DT, Pereira AKDS, Scheidt GN, Pereira DH (2019) Plant and bacterial cellulose: production, chemical structure, derivatives and applications. Orbital Electron J Chem 11:321–329. https://doi.org/10.17807/orbital.v11i5.1349
Ribeiro IHS, Reis DT, Pereira DH (2019) A DFT-based analysis of adsorption of Cd2+, Cr3+, Cu2+, Hg2+, Pb2+, and Zn2+, on vanillin monomer: a study of the removal of metal ions from effluents. J Mol Model 25:267. https://doi.org/10.1007/s00894-019-4151-z
Kanmani P, Aravind J, Kamaraj M et al (2017) Environmental applications of chitosan and cellulosic biopolymers: a comprehensive outlook. Bioresour Technol 242:295–303. https://doi.org/10.1016/j.biortech.2017.03.119
Ren N-Q, Zhao L, Chen C et al (2016) A review on bioconversion of lignocellulosic biomass to H2: key challenges and new insights. Bioresour Technol 215:92–99. https://doi.org/10.1016/j.biortech.2016.03.124
Rajeswari A, Amalraj A, Pius A (2016) Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J Water Process Eng 9:123–134. https://doi.org/10.1016/j.jwpe.2015.12.002
An B, Jung K-Y, Zhao D et al (2014) Preparation and characterization of polymeric ligand exchanger based on chitosan hydrogel for selective removal of phosphate. React Funct Polym 85:45–53. https://doi.org/10.1016/j.reactfunctpolym.2014.10.003
Isik M, Sardon H, Mecerreyes D (2014) Ionic liquids and cellulose: dissolution, chemical modification and preparation of new cellulosic materials. Int J Mol Sci 15:11922–11940. https://doi.org/10.3390/ijms150711922
Silva Filho EC, Santos Júnior LS, Silva MMF et al (2013) Surface cellulose modification with 2-aminomethylpyridine for copper, cobalt, nickel and zinc removal from aqueous solution. Mater Res 16:79–84. https://doi.org/10.1590/S1516-14392012005000147
Reis DT, Ribeiro IHS, Pereira DH (2019) DFT study of the application of polymers cellulose and cellulose acetate for adsorption of metal ions (Cd2+, Cu2+ and Cr3+) potentially toxic. Polym Bull. https://doi.org/10.1007/s00289-019-02926-5
Liang S, Guo X, Feng N, Tian Q (2010) Effective removal of heavy metals from aqueous solutions by orange peel xanthate. Trans Nonferr Met Soc China 20:s187–s191. https://doi.org/10.1016/S1003-6326(10)60037-4
Beyki MH, Bayat M, Miri S et al (2014) Synthesis, Characterization, and silver adsorption property of magnetic cellulose xanthate from acidic solution: prepared by one step and biogenic approach. Ind Eng Chem Res 53:14904–14912. https://doi.org/10.1021/ie501989q
Biswal DR, Singh RP (2004) Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr Polym 57:379–387. https://doi.org/10.1016/j.carbpol.2004.04.020
Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer, Berlin
Castagnetto JM, Hennessy SW, Roberts VA et al (2002) MDB: the metalloprotein database and browser at the scripps research institute. Nucl Acids Res 30:379–382. https://doi.org/10.1093/nar/30.1.379
Nascimento RF do, Lima ACA de, Vidal CB et al (2014) Adsorção: aspectos teóricos e aplicações ambientais. Impresso no Brasil. Ceará
do Nascimento RF, Sousa Neto VDO, Melo DDQ (2014) Uso de bioadsorventes lignocelulósicos na remoção de poluentes de efluentes aquosos. Impresso no Brasil. Ceará
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184. https://doi.org/10.1002/jcb.26234
Galaris D, Evangelou A (2002) The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Crit Rev Oncol/Hematol 42:93–103. https://doi.org/10.1016/S1040-8428(01)00212-8
Monalisa M, Kumar PH (2013) Effect of ionic and chelate assisted hexavalent chromium on mung bean seedlings (Vigna radiata L, wilczek, Var k-851) during seedling growth. J Stress Physiol Biochem, vol 9. https://cyberleninka.ru/article/n/effect-of-ionic-and-chelate-assisted-hexavalent-chromium-on-mung-bean-seedlings-vigna-radiata-l-wilczek-var-k-851-during-seedling-growth
Rodríguez MC, Barsanti L, Passarelli V et al (2007) Effects of chromium on photosynthetic and photoreceptive apparatus of the alga Chlamydomonas reinhardtii. Environ Res 105:234–239. https://doi.org/10.1016/j.envres.2007.01.011
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://pubs.acs.org/doi/abs/10.1021/jp810292n
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb M A, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralt, JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, Revision C.01. Gaussian, Inc, Wallingford
Dennington R, Keith T, Millam J (2009) GaussView, Version 5. Semichem Inc, Shawnee Mission
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80:1943–1960. https://doi.org/10.1063/1.446956
Keith TA, Bader RFW, Aray Y (1996) Structural homeomorphism between the electron density and the virial field. Int J Quantum Chem 57:183–198. https://doi.org/10.1002/(SICI)1097-461X(1996)57:2%3c183:AID-QUA4%3e3.0.CO;2-U
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128:1527–1536. https://doi.org/10.1007/s12039-016-1172-3
Popelier PLA (1999) Quantum molecular similarity, 1, BCP space. J Phys Chem A 103:2883–2890. https://doi.org/10.1021/jp984735q
Keith TA (2017) AIMAll (Version 17.11.14). TK Gristmill Software, Overland Park KS, USA (aim.tkgristmill.com)
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://pubs.acs.org/doi/abs/10.1021/ja00905a001
Vasconcellos MLAA (2014) A teoria de pearson para a disciplina de Química Orgânica: um exercício prático e teórico aplicado em sala de aula. Quím Nova 37(1):171–175. https://doi.org/10.1590/S0100-40422014000100029
Acknowledgements
The authors acknowledge funding from CAPES (Coordination of Improvement of Higher Education Personnel—Brazil), Funding Code 001 CAPES. Pereira DH acknowledges the Center for Computational Engineering and Sciences (Financial support from FAPESP Fundação de Amparo à Pesquisa, Grant 2013/08293-7, and Grant 2017/11485-6), the National Center for High Performance Processing (Centro Nacional de Processamento de Alto Desempenho—CENAPAD) in São Paulo and UNICAMP (Universidade Estadual de Campinas), for computational resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Festschrift in honor of Prof. Fernando R. Ornellas” Guest Edited by Adélia Justino Aguiar Aquino, Antonio Gustavo Sampaio de Oliveira Filho and Francisco Bolivar Correto Machado.
Rights and permissions
About this article
Cite this article
Reis, D.T., de Aguiar Filho, S.Q., Grotto, C.G.L. et al. Carboxymethylcellulose and cellulose xanthate matrices as potential adsorbent material for potentially toxic Cr3+, Cu2+ and Cd2+metal ions: a theoretical study. Theor Chem Acc 139, 96 (2020). https://doi.org/10.1007/s00214-020-02610-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02610-2