Abstract
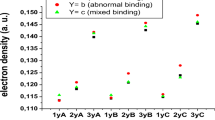
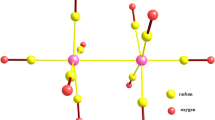
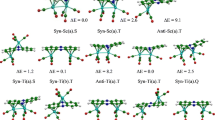
The calculations of bimetallic complexes of the type (X)[(Ind)M2(L)2] (M = Ni, Pd, L = CO, (PEt3) and X = Allyl, Cp and indenyl) have been done using two DFT functionals, namely BP86 and B3LYP*. The allyl, Cp and indenyl ligands adopt the same η3-coordination mode with a π bond and can be considered to be isolobal, while the chloride acts as σ- and π-donor. The computed structural and energetic parameters and energy decomposition yield chemically useful information. We report that the metal–metal bond distances are slightly sensitive to the electron donation and electron π-backdonation as the isolobal prediction suggests. Changing the metal from Ni to Pd has the result of increasing the metal–metal bond distance, decreasing the natural population of Pd and the weakness interactions between the X− ligand and the [(Ind)M2(L)2]+ fragment. The results showed that the four ligands behave quite similarly in terms of bonding, coordination mode and donation and π-backdonation properties highlighted by the orbitals’ populations and the energy decomposition. However, the strength of interactions can be classified as follows: Cl < Cp ≈ Ind < Allyl. In all the complexes studied, the M22+ moiety adopts a single metal–metal bonding attributing the 16-MVE configuration to each M(I) cation.







Similar content being viewed by others
References
Lin S, Agapie T (2011) Cross-coupling chemistry at mononuclear and dinuclear nickel complexes. Synlett 01:1–5
Hazari N, Hruszkewycz DP (2016) Dinuclear Pd(I) complexes with bridging allyl and related ligands. Chem Soc Rev 45:2871–2899
Paton RS, Brown JM (2012) Dinuclear palladium complexes-precursors or catalysts. Angew Chem Int Ed Engl 51:10448–10450
Zimmermann P, Limberg C (2017) Activation of small molecules at nickel (I) moieties. J Am Chem Soc 139:4233–4242
Bonney KJ, Schoenebeck F (2014) Experiment and computation: a combined approach to study the reactivity of palladium complexes in oxidation states 0 to IV. Chem Soc Rev 43:6609–6638
Liu Q, Dong X, Li J, Xiao J, Dong Y, Liu H (2015) Recent advances on palladium radical involved reactions. ACS Catal 5:6111–6137
Werner H (1981) Novel types of metal–metal bonded complexes containing allyl and cyclopentadienyl bridging ligands. Adv Organomet Chem 19:155–182
Murahashi T, Kurosawa H (2002) Organopalladium complexes containing palladium–palladium bonds. Coord Chem Rev 231:207–228
Hazari N, Hruszkewycz DP, Wu J (2011) Pd(I)-bridging allyl dimers: a new system for the catalytic functionalization of carbon dioxide. Synlett 13:1793–1797
Inatomi T, Koga Y, Matsubara K (2018) Dinuclear nickel (I) and palladium (I) complexes for highly active transformations of organic compounds. Molecules 23(1):140
Hazari N, Hruszkewycz DP (2016) Dinuclear Pd(I) complexes with bridging allyl and related ligands. Chem Soc Rev 45(10):2871–2899
Werner H et al (1975) Preparation and structure of novel binuclear palladium complexes containing a metal–metal bond: μ-(η-C5H5)-μ-(η-C4H7)Pd2L2. Angew Chem Int Ed Engl 14(3):185–186
Werner H, Kühn A, Tune DJ, Krüger C, Brauer DJ, Sekutowski JC, Tsay YH (1977) Untersuchungenzur Reaktivität von Metall-π-Komplexen, XXII. Sandwichartiggebaute (Pd–Pd)-Zweikernkomplexemitbrückenbildenden Cyclopentadienyl-und Allyl-Liganden. Chem Beri 110:1763–1775
Kühn A, Werner H (1979) Untersuchungenzurreaktivität von metall-π-komplexen: XXIX. Synthesewege und reaktivität von (Pd–Pd)-und (Pt–Pt)-zweikernkomplexen des typs (μ-C5H5)(μ-allyl)M2L2. J Organomet Chem 179:421–438
Werner H, Kühn A (1977) A general method for the synthesis of sandwich-type complexes with a Pd–Pd or Pt–Pt bond. Angew Chem Int Ed Engl 16:412–413
Werner H, Kraus HJ (1979) Synthesis and structural dynamics of bis(cyclopentadienyl) phosphanepalladium complexes. Angew Chem Int Ed Engl 18:948–949
Norton DM, Mitchell EA, Botros NR, Jessop PG, Baird MC (2009) A superior precursor for palladium (0)-based cross-coupling and other catalytic reactions. J Org Chem 74:6674–6680
Chalkley MJ, Guard LM, Hazari N, Hofmann P, Hruszkewycz DP, Schmeier TJ, Takase MK (2013) Synthesis, electronic structure, and reactivity of palladium (I) dimers with bridging allyl, cyclopentadienyl, and indenyl ligands. Organometallics 32:4223–4238
Melvin PR, Nova A, Balcells D, Dai W, Hazari N, Hruszkewycz DP, Tudge MT et al (2015) Design of a versatile and improved precatalyst scaffold for palladium-catalyzed cross-coupling:(η3-1-tBu-indenyl) 2 (μ-Cl) 2Pd2. ACS Catal 5:3680–3688
Tanase T, Nomura T, Fukushima T, Yamamoto Y, Kobayashi K (1993) Synthesis and characterization of a binuclear palladium (I) complex with bridging η3-indenyl ligands, Pd2 (μ-η3-indenyl)2(isocyanide)2, and its transformation to a tetranuclear palladium (I) cluster of isocyanides, Pd4 (μ-acetate) 4 (μ-isocyanide) 4. Inorg Chem 32:4578–4584
Tanase T, Nomura T, Yamamoto Y, Kobayashi K (1991) Synthesis and characterization of binuclear palladium complexes of isocyanides with novel bridging η3-indenyl ligands. Crytal structure of [Pd2(μ-η3-indenyl)2(RNC)2](R = 2, 6-dimethylphenyl). J Organomet Chem 410:C25–C28
Dai W, Chalkley MJ, Brudvig GW, Hazari N, Melvin PR, Pokhrel R, Takase MK (2013) Synthesis and properties of NHC-supported palladium (I) dimers with bridging allyl, cyclopentadienyl, and indenyl ligands. Organometallics 32:5114–5127
Tanase T, Fukushima T, Nomura T, Yamamoto Y, Kobayashi K (1994) Synthesis and characterization of binuclear palladium (I) complexes of isocyanides with phenyl-substituted cyclopentadienyl and tris (pyrazol-1-yl) borate ligands. Inorg Chem 33(1):32–39
Sui-Seng C, Enright GD, Zargarian D (2006) New palladium (II)-(η3/5 or η1-Indenyl) and dipalladium (I)-(μ, η3-Indenyl) complexes. J Am Chem Soc 128:6508–6519
Michael P, Mingos D (1996) Synthesis and structural characterisation of [Pd 2 (Á-Br) 2 (PBu t 3) 2], an example of a palladium(I)–palladium(I) dimer. J Chem Soc Dalton Trans 23:4313–4314
Proutiere F, Lyngvi E, Aufiero M, Sanhueza IA, Schoenebeck F (2014) Combining the reactivity properties of PCy3 and PtBu3 into a single ligand, P(iPr)(tBu)2. Reaction via mono- or bisphosphine palladium(0) centers and palladium(I) dimer formation. Organometallics 33:6879–6884
Beck R, Johnson SA (2013) Dinuclear Ni(I)–Ni(I) complexes with syn-facial bridging ligands from Ni(I) precursors or Ni(II)/Ni(0) comproportionation. Organometallics 32:2944–2951
Wang H, Sun S, Wang H, King RB (2016) Binuclear cyclooctatetraene–iron carbonyl complexes: examples of fluxionality and valence tautomerism. N J Chem 40:1521–1528
Jin R, Chen X, Du Q, Feng H, Xie Y, King RB, Schaefer HF (2016) Binuclear iron carbonyl complexes of thialene. RSC Adv 6:82661–82668
Wang H, Sun Z, Xie Y, King RB, Schaefer HF III (2010) Unsaturation and variable hapticity in binuclear azulene iron carbonyl complexes. Organometallics 29:630–641
Zendaoui SM, Zouchoune B (2013) Molecular properties and electronic structure of phenazine ligand in binuclear molybdenum and manganese metal complexes: a density functional theory study. Polyhedron 51:123–131
Zendaoui SM, Saillard JY, Zouchoune B (2016) Ten-electron donor indenyl anion in binuclear transition-metal sandwich complexes: electronic structure and bonding analysis. Chem Select 1(5):940–948
Zouchoune B, Zendaoui SM, Saillard JY (2018) Why is bis-indenylchromium a dimer? A DFT investigation. J Organomet Chem 858:47–52
Drideh S, Zouchoune B, Zendaoui SM, Saillard JY (2018) Electronic structure and structural diversity in indenyl in heterobinuclear transition-metal half-sandwich complexes. Theor Chem Acc 137(7):99
Nemdili H, Zouchoune B, Zendaoui SM, Ferhati A (2019) Structural, bonding and redox properties of 34-electron bimetallic complexes and their oxidized 32-and 33-electron and reduced 35-and 36-electron derivatives containing the indenyl fused π-system: a DFT overview. Polyhedron 160:219–228
Korichi H, Zouchoune F, Zendaoui SM, Zouchoune B, Saillard JY (2010) The coordination chemistry of azulene: a comprehensive DFT investigation. Organometallics 29:1693–1706
Merzoug M, Zouchoune B (2014) Coordination diversity of the phenazine ligand in binuclear transition metal sandwich complexes: theoretical investigation. J Organomet Chem 770:69–78
Bensalem N, Zouchoune B (2016) Coordination capabilities of anthracene ligand in binuclear sandwich complexes: DFT investigation. Struct Chem 27(6):1781–1792
Naili N, Zouchoune B (2018) Structural diversity of homobinuclear transition metal complexes of the phenazine ligand: theoretical investigation. Struct Chem 29(3):725–739
ADF2016, SCM (2016) Theoretical chemistry. Vrije Universiteit, Amsterdam
Baerends EJ, Ellis DE, Ros PJCP (1973) Self-consistent molecular Hartree–Fock Slater calculations I. The computational procedure. Chem Phys 2:41–51
TeVelde G, Baerends EJ (1992) Numerical integration for polyatomic systems. J Comput Phys 99:84–98
Guerra CF, Snijders JG, teVelde GT, Baerends EJ (1998) Towards an order-N DFT method. Theor Chem Acc 99(6):391–403
Bickelhaupt FM, Baerends EJ (2000) Kohn–Sham density functional theory: predicting and understanding chemistry. Rev Comput Chem 15:1–86
TeVelde GT, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, van Gisbergen SJ, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931–967
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211
Becke AD (1986) Density functional calculations of molecular bond energies. J Chem Phys 84:4524–4529
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33:8822–8824
Perdew JP (1986) Erratum: density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 34:7406
Salomon O, Reiher M, Hess BA (2002) Assertion and validation of the performance of the B3LYP* functional for the first transition metal row and the G2 test set. J Chem Phys 117:4729–4737
Becke AD (1993) Becke’s three parameter hybrid method using the LYP correlation functional. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Versluis L, Ziegler T (1988) The determination of molecular structures by density functional theory. The evaluation of analytical energy gradients by numerical integration. J Chem Phys 88:322–328
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on main group molecules. J Chem Phys 96:9005–9012
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on transition-metal carbonyls. J Phys Chem 96:6937–6941
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor–acceptor perspective. Cambridge University Press, Cambridge
Weinhold F, Glendening ED (2001) NBO 5.0 program manual: natural bond orbital analysis programs. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison
Zouchoune B, Saiad A (2018) Ligands’ σ-donation and π-backdonation effects on metal-metal bonding in trinuclear [M3(Tr)2(L)3]2 + (M = Fe, Ni, Pd, Pt, Tr = Tropylium and L = CO, HCN and C2H4) sandwich compounds: theoretical investigation. Inorg Chim Acta 473:204–215
Zouchoune B (2018) Stability and possible multiple metal-metal bonding in tetranuclear sandwich complexes of cyclooctatetraene ligand. Struct Chem 29:937–945
Fadli S, Zouchoune B (2017) Coordination chemistry and bonding analysis of tetranuclear transition metal pyrene sandwich complexes. Struct Chem 28:985–997
Benmachiche A, Zouchoune B (2019) Coordination and ligands’ effects in trinuclear [Pd3(COT)2(L)]2 + (L = H2O, CO, N2, HCN, HNC, NH3, PH3, PCl3, PF3, CS, CH2) sandwich complexes of cyclooctatetraene: theoretical investigation. Struct Chem 30:1–8
Yamamoto Y, Yamazaki H (1985) Dichlorotetrakis (isocyanide) dipalladium (I) containing a metal–metal bond. Bull Chem Soc Jpn 58:1843–1844
Yamamoto Y, Yamazaki H (1986) Studies on interactions of isocyanides with transition-metal complexes. X-ray crystal structure of dichlorobis (2, 6-dimethylphenyl isocyanide) bis (pyridine) dipalladium. Unusual carbon-nitrogen-carbon bond angles and infrared spectrum in bridging isocyanide groups. Inorg Chem 25(18):3327–3329
Holloway RG, Penfold BR, Colton R, McCormick MJ (1976) Crystal and molecular structure of bis-µ-(bisdiphenylphosphinomethane)-dibromodipalladium (Pd-Pd), a compound containing palladium (I). J Chem Soc Chem Commun 12:485–486
Kullberg ML, Lemke FR, Powell DR, Kubiak CP (1985) Palladium–palladium. Sigma bonds supported by bis(dimethylphosphino)methane (dmpm). Synthetic, structural, and Raman studies of Pd2X2(dmpm)2 (X = Cl, Br, OH). Inorg Chem 24:3589–3593
Morokuma K (1971) Molecular orbital studies of hydrogen bonds. III. C=O···H–O hydrogen bond in H2CO···H2O and H2CO···2H2O. J Chem Phys 55:1236–1244
Ziegler T, Rauk A (1979) Carbon monoxide, carbon monosulfide, molecular nitrogen, phosphorus trifluoride, and methyl isocyanide as. sigma. donors and. pi. acceptors. A theoretical study by the Hartree–Fock–Slater transition-state method. Inorg Chem 18:1755–1759
Ziegler T, Rauk A (1979) A theoretical study of the ethylene-metal bond in complexes between copper (1 +), silver (1 +), gold (1 +), platinum (0) or platinum (2 +) and ethylene, based on the Hartree–Fock–Slater transition-state method. Inorg Chem 18:1558–1565
Acknowledgements
The authors acknowledge the Algerian MESRS (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique) and DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique) for financial support.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
This work is dedicated to Doctor Jean-François Halet on the occasion of his 60th birthday.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mecheri, S., Zouchoune, B. & Zendaoui, SM. Bonding and electronic structures in dinuclear (X)[(Ind)M2L2] complexes (M = Ni, Pd, L = CO, PEt3, X = Cl, Allyl, Ind = indenyl, Cp = cyclopentadienyl): analogy between four-electron donor ligands. Theor Chem Acc 139, 12 (2020). https://doi.org/10.1007/s00214-019-2526-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2526-y