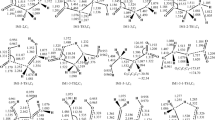
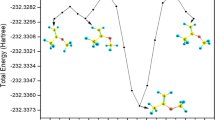
Abstract
The detailed reaction mechanism of 3-chloro-2-methyl-1-propene (3CMP) with OH radical was investigated by employing highly accurate electronic structure calculations and kinetic modelling. Due to the unsaturated structure of 3CMP, it is highly reactive in the troposphere with OH radical. The fate of the so-formed alkyl radical intermediates and intermediate adducts in the favourable pathways is determined by its reaction with other atmospheric oxidants, such as HO2, NO and NO2 radicals. The rate constants computed within the temperature range of 200–1000 K for the favourable hydrogen atom abstraction and OH radical addition reactions are in reasonable agreement with the available experimental values. The oxidation of 3CMP results in the formation of stable chlorinated products, such as chloroacetone and formyl chloride, which are identified experimentally. These products may be transported to the stratosphere which will affect the ozone layer.
















Similar content being viewed by others
References
Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN (2009) New Phytol 183:27–51
Koppmann R (2007) Volatile organic compounds in the atmosphere. Wiley, London
Kodavanti PRS, Senthilkumar K, Loganathan G (2008) Int Encycl Public Health 4:686–693
Adriaens P, Gruden C, McCormick M (2003) Treatise Geochem 9:612
Budnik LT, Fahrenholtz S, Kloth S, Baur X (2010) J Environ Monit 12:936–942
Kittrell JR, Quinlan CW, Eldridge JW (1991) J Air Waste Manag Assoc 41:1129–1133
Atkinson R, Arey J (2003) Chem Rev 103:4605–4638
Finlayson-Pitts BJ, Pitts JN Jr (1999) Chemistry of the upper and lower atmosphere: theory, experiments, and applications. Elsevier, Amsterdam
Appendix C (1994) Chlorinated alkenes. Regul Toxicol Pharmacol 20:S757–S818
Carcinogens (2011) U.S. Department of Health and Human Services Secretary Kathleen Sebelius released the 12th report on carcinogens
Rivela C, Gibilisco RG, Teruel MA (2015) J Phys Organ Chem 28:480–484
Zhang Q, Chen Y, Tong S, Ge M, Shenolikar J, Johnson MS, Wang Y, Tsona NT, Mellouki A, Du L (2017) Atmos Environ 170:12–21
Yujing M, Mellouki A (2001) Phys Chem Chem Phys 3:2614–2617
Begum SS, Gour NK, Baruah SD, Deka RC (2018) Mol Phys 117:1–9
Zhao Y, Truhlar DG (2008) Acc Chem Res 41:157–167
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Karton A, Tarnopolsky A, Schatz GC, Martin JML (2008) J Phys Chem A 112:12868–12886
Xie HB, Li C, He N, Wang C, Zhang S, Chen J (2014) Environ Sci Technol 48:1700–1706
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615–6620
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Purvis GD, Bartlett RJ (1982) J Chem Phys 76:1910–1918
Vereecken L, Francisco JS (2012) Chem Soc Rev 41:6259–6293
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) J Chem Phys 110:2822–2827
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (2000) J Chem Phys 112:6532–6542
Frisch MJ, Trucks GE, Schlegel HB, Scuseria GE, RobbMA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al. (2009) Gaussian 09
Garrett BC, Truhlar DG (1979) J Am Chem Soc 101:4534–4548
Garrett BC, Truhlar DG (1979) J Chem Phys 70(4):1593–1598
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730–1748
Zheng J, Zhang S, Corchado J, Chuang Y, Coitino E, Ellingson B, Truhlar D (2015) GAUSSRATE. University of Minnesota, Minneapolis
Zheng J, Zhang S, Lynch BJ, Corchado JC, Chuang YY, Fast P, Hu W, Liu YP, Lynch G, Nguyen K (2010) POLYRATE, version 2010
Bhuvaneswari R, Sandhiya L, Senthilkumar K (2017) J Phys Chem A 121:6028–6035
Jacob DJ, Potter T, Colman B, Fishman J, Hill MA (2003) The oxidizing power of the atmosphere. In: Handbook of weather, climate and water, pp 29–46
Arey J, Atkinson R, Zielinska B, McElroy PA (1989) Environ Sci Technol 23:321–327
Bunce NJ, Dryfhout HG (1992) Can J Chem 70:1966–1970
Orlando JJ, Tyndall GS (2012) Chem Soc Rev 41:6294–6317
Orlando JJ, Tyndall GS, Wallington TJ (2003) Chem Rev 103:4657–4690
Lightfoot PD, Cox RA, Crowley JN, Destriau M, Hayman GD, Jenkin ME, Moortgat GK, Zabel F (1992) Atmos Environ 26:1805–1961
Zhang J, Dransfield T, Donahue NM (2004) J Phys Chem A 108:9082–9095
Li Y, Francisco JS (2001) J Chem Phys 114:2879–2882
Zhou M, Ma R, Yuan D, Chen M (2009) J Phys Chem A 113:2826–2830
Kleijn R, Elshkaki A, De Koning A, Tukker A (2001) Literature study on degradation products of known emissions
Tanaka N, Yamagishi S, Nishikiori H (2013) Comput Theor Chem 1020:108–112
Acknowledgements
The authors are thankful to UGC and Department of Science and Technology (DST), India, for funding to establish the high-performance computing facility under the SAP and PURSE programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
214_2019_2518_MOESM1_ESM.doc
The TST, CVT, TST (SCT) and CVT (SCT) rate constants (cm3 molecule−1 s−1) for the initial H-abstraction reactions (R2 and R3) and OH-addition reactions (R4 and R5) of 3CMP with OH radical are given in Table S1. Relative energy, ΔETot (kcal/mol), enthalpy, ΔH298 (kcal/mol), and Gibbs free energy, ΔG298 (kcal/mol), for the reactions of radical intermediates, I2, I3, 45 and I5, calculated at M06-2X, ωB97XD and CCSD(T) level of theories are summarized in Table S2, S3, S4 and S5. (DOC 1109 kb)
Rights and permissions
About this article
Cite this article
Bhuvaneswari, R., Senthilkumar, K. A comprehensive quantum chemical study on the mechanism and kinetics of atmospheric reactions of 3-chloro-2-methyl-1-propene with OH radical. Theor Chem Acc 139, 2 (2020). https://doi.org/10.1007/s00214-019-2518-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2518-y