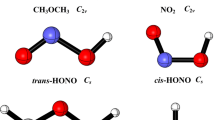
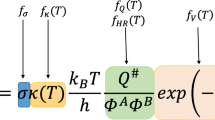Abstract
Hydrogen abstraction from dimethyl ether (DME) by nitric dioxide (NO2) has been considered to be very sensitive to the DME ignition behavior. Thermal rate coefficients for the reaction of DME with NO2 are computed by employing the multi-structural canonical variational transition-state theory with multidimensional tunneling (MS-CVT/MT) at temperatures from 200 to 3000 K. The B2PLYP/TZVP theoretical level has been verified in the first part of this study against experiment for geometries of stationary points and against accurate CCSD(T)/CBS and CCSD(T)/6-311+G(2df,2p) computations for the energetics related to the title reaction, and then the compound CCSD(T)/6-311+G(2df,2p)//B2PLYP/TZVP computations are performed here to obtain the properties of all stationary points and the geometries, energies, gradients, and Hessians of non-stationary points along each reaction minimum-energy pathway. It has been proposed that the internal rotations in the transition structures are anharmonic and strongly coupled to each other to produce multiple conformations whose contributions are included in their partition function approximations. The previous evaluations for these rate coefficients based on transition-state theory with one-dimensional tunneling corrections and one-dimensional hindered rotor treatments of torsions are essentially distinct from our MS-CVT/SCT results where the critical effects of corner cutting due to small reaction-path curvature are seriously treated via multidimensional small-curvature tunneling (SCT) treatment and torsions are explicitly considered by multistructural treatments, and thus the MS-CVT/SCT rate coefficients are proposed particularly to refine the current kinetic model for DME combustion.








Similar content being viewed by others
References
Kohse-Höinghaus K, Oßwald P, Cool TA, Kasper T, Hansen N, Qi F, Westbrook CK, Westmoreland PR (2010) Biofuel combustion chemistry: from ethanol to biodiesel. Angew Chem Int Ed 49:3572–3597
Arcoumanis C, Bae C, Crookes R, Kinoshita E (2008) The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: a review. Fuel 87:1014–1030
Tanaka S, Ayala F, Keck JC, Heywood JB (2003) Two-stage ignition in HCCI combustion and HCCI control by fuels and additives. Combust Flame 132:219–239
El-Asrag HA, Ju Y (2014) Direct numerical simulations of NOx effect on multistage autoignition of DME/air mixture in the negative temperature coefficient regime for stratified HCCI engine conditions. Combust Flame 161:256–269
Imtenan S, Varman M, Masjuki HH, Kalam MA, Sajjad H, Arbab MI, Rizwanul Fattah IM (2014) Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: a review. Energy Convers Manage 80:329–356
Reitz RD, Duraisamy G (2015) Review of high efficiency and clean reactivity controlled compression ignition (RCCI) combustion in internal combustion engines. Prog Energy Combust 46:12–71
Alzueta MU, Muro J, Bilbao R, Glarborg P (1999) Oxidation of dimethyl ether and its interaction with nitrogen oxides. Israel J Chem 39:73–86
Dagaut P, Lucheke J, Cathonnet M (2001) The low temperature oxidation of DME and mutual sensitization of the oxidation of DME and nitric oxide: experimental and detailed kinetic modeling. Combust Sci Technol 165:61–84
Ye W, Shi JC, Zhang RT, Wu XJ, Zhang X, Qi ML, Luo SN (2016) Experimental and kinetic modeling study of CH3OCH3 ignition sensitized by NO2. Energy Fuels 30:10900–10908
Xiao CX, Yan N, Zou M, Hou SC, Kou Y, Liu W, Zhang S (2006) NO2-catalyzed deep oxidation of methanol: experimental and theoretical studies. J Mol Catal A Chem 252:202–211
Shang YL, Shi JC, Fang LM, Feng QG, Wang HY, Luo SN (2018) Theoretical investigation on hydrogen abstraction by NO2 from symmetric ethers (CH3)2xO (x = 1–4). J Phys Chem A 122:6829–6841
Yu T, Zheng J, Truhlar DG (2011) Multi-structural variational transition state theory. Kinetics of the 1,4-hydrogen shift isomerization of the pentyl radical with torsional anharmonicity. Chem Sci 2:2199–2213
Garrett BC, Truhlar DG (1979) Generalized transition state theory. Bond energy-bond order method for canonical variational calculations with application to hydrogen atom transfer reactions. J Am Chem Soc 101:4534–4548
Truhlar DG, Garrett BC (1980) Variational transition-state theory. Acc Chem Res 13:440–448
Zheng J, Yu T, Papajak E, Alecu IM, Mielke SL, Truhlar DG (2011) Practical methods for including torsional anharmonicity in thermochemical calculations on complex molecules: the internal-coordinate multi-structural approximation. Phys Chem Chem Phys 13:10885–10907
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) Improved treatment of threshold contributions in variational transition-state theory. J Phys Chem 84:1730–1748
Liu YP, Lynch GC, Truong TN, Lu DH, Truhlar DG, Garrett BC (1993) Molecular modeling of the kinetic isotope effect for the [1,5] sigmatropic rearrangement of cis-1,3-Pentadiene. J Am Chem Soc 115:2408–2415
Zheng J, Truhlar DG (2010) Kinetics of hydrogen-transfer isomerizations of butoxyl radicals. Phys Chem Chem Phys 12:7782–7793
Corchado JC, Chuang YY, Fast PL, Hu WP, Liu YP, Lynch GC, Nguyen KA, Jackels CF, Ramos AF, Ellingson BA et al (2007) Polyrate 9.7. University of Minnesota, Minneapolis
Grimme S (2006) Semiempirical hybrid density functional with perturbative second-order correlation. J Chem Phys 124:034108
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100:5829–5835
Hratchian HP, Schlegel HB (2004) Accurate reaction paths using a Hessian based predictor–corrector integrator. J Chem Phys 120:9918–9924
Hratchian HP, Schlegel HB (2005) Using Hessian updating to increase the efficiency of a hessian based predictor-corrector reaction path following method. J Chem Theory Comput 1:61–69
Guan Y, Li Y, Zhao L, Song Y, Ma H, Song J (2016) Hydrogen transfer between dimethyl ether and the methoxy radical: understanding and kinetic modeling with anharmonic torsions. Comput Theor Chem 1089:43–53
Pitzer KS, Gwinn WD (1942) Energy levels and thermodynamic functions for molecules with internal rotation I. Rigid frame with attached tops. J Chem Phys 10:428–440
Pitzer KS (1946) Energy levels and thermodynamic functions for molecules with internal rotation II. Unsymmetrical tops attached to a rigid frame. J Chem Phys 14:239–243
Fernández-Ramos A, Ellingson BA, Meana-Pañeda R, Marques JMC, Truhlar DG (2007) Symmetry numbers and chemical reaction rates. Theor Chem Acc 118:813–826
Guan Y, Gao J, Song Y, Li Y, Ma H, Song J (2017) Variational effect and anharmonic torsion on kinetic modeling for initiation reaction of dimethyl ether combustion. J Phys Chem A 121:1121–1132
Dayma G, Ali KH, Dagaut P (2007) Experimental and detailed kinetic modeling study of the high pressure oxidation of methanol sensitized by nitric oxide and nitrogen dioxide. Proc Combust Inst 31:411–418
Rasmussen CL, Rasmussen AE, Glarborg P (2008) Sensitizing effects of NOx on CH4 oxidation at high pressure. Combust Flame 154:529–545
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21606178).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
214_2019_2516_MOESM1_ESM.doc
Part I. Optimized geometries, rotational constants, and unscaled frequencies for all TSa, TSb, and TSc structures using B2PLYP/TZVP level. The τ-i, τ-ii, τ-iii, and τ-iv potentials along their torsion coordinates fitted to the Fourier series of all TSa, TSb, and TSc structures are also included. Part II. Thermal rate coefficients for the channels R(a), R(b), and R(c) of the reaction of dimethyl ether with nitric dioxide. (DOC 1507 kb)
Rights and permissions
About this article
Cite this article
Guan, Y., Liu, R., Lou, J. et al. Computational investigation on the reaction of dimethyl ether with nitric dioxide. II. Detailed chemical kinetic modeling. Theor Chem Acc 139, 1 (2020). https://doi.org/10.1007/s00214-019-2516-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2516-0