Abstract
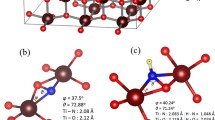
Nitroxide radical redox-couple compounds have the potentials to be applied as a redox couple in dye-sensitized solar cell applications since their standard reduction potential reached almost 1 V. Unfortunately, many reports revealed the limitations of nitroxide in dye-sensitized solar cell applications. This study investigates the interaction between several nitroxide radical redox couples with anatase surface. The Ti14O31H6 cluster was built as a model of anatase surface. The calculation results show that the limitation of the nitroxide radical redox-couple compounds was laid on the high charge transfer on the TiO2 surface. The high charge transfer indicates that the charge recombination from the redox-couple species to the semiconductor is preferable and is not beneficial for cell efficiency. The calculation also figures that almost the observed nitroxide radical compounds shifted the TiO2 conduction band positively to the − 2.5 eV. This finding proved to us that it is important to choose the sensitizer which has a LUMO above − 2.5 eV to avoid the fail of charge injection.





Similar content being viewed by others
References
Lewis NS (2016) Research opportunities to advance solar energy utilization. Science 351:aad1920
Kabir E, Kumar P, Kumar S, Adelodun AA, Kim KH (2018) Solar energy: potential and future prospects. Renew Sustain Energy Rev 82:894–900
Vittal R, Ho KC (2017) Zinc oxide based dye-sensitized solar cells: a review. Renew Sustain Energy Rev 70:920–935
Duvva N, Chilakamarthi U, Giribabu L (2017) Recent developments in tetrathiafulvalene and dithiafulvalene based metal-free organic sensitizers for dye-sensitized solar cells: a mini-review. Sustain Energy Fuels 1:678–688
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Ito S, Murakami TN, Comte P, Liska P, Gratzel C, Nazeeruddin MK, Gratzel M (2008) Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%. Thin Solid Films 516:4613–4619
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110(11):6595–6663
Martsinovich N, Troisi A (2011) Theoretical studies of dye-sensitised solar cells: from electronic structure to elementary processes. Energy Environ Sci 4:4473–4495
Agrawal S, English NJ, Thampi KR, MacElroy JMD (2012) Perspectives on ab initio molecular simulation of excited-state properties of organic dye molecules in dye-sensitised solar cells. Phys Chem Chem Phys 14:12044–12056
Hamann TW (2012) The end of iodide? Cobalt complex redox shuttles in DSSCs. Dalton Trans 41:3111–3115
Saygili Y, Soderberg M, Pellet N, Giordano F, Cao Y, Munoz-Garcia AB, Zakeeruddin SM, Vlachopoulos N, Pavone M, Boschloo G, Kavan L, Moser JE, Gratzel M, Hagfeldt A, Freitag M (2016) Copper bipyridyl redox mediators for dye-sensitized solar cells with high photovoltage. J Am Chem Soc 138:15087–15096
Kusumawati Y, Massin J, Olivier C, Toupance T, Ivansyah AL, Martoprawiro MA, Prijamboedi B, Radiman CL, Pauporte T (2017) Combined computational and experimental study of carbazole dyes for iodide- and cobalt-based ZnO DSSCs. J Photochem Photobiol Chem 341:69–77
Mosconi E, Yum JH, Kessle F, Garcia CJG, Zuccaccia C, Cinti A, Nazeeruddin MK, Gratzel M, De Angelis F (2012) Cobalt electrolyte/dye interactions in dye-sensitized solar cells: a combined computational and experimental study. J Am Chem Soc 134:19438–19453
Erden I, Faideci MA, Erdönmez S (2018) Dye-sensitized solar cell performance of a cobalt(III/II) redox mediator with the 4,5-diazafluoren-9-one ligand. Transit Met Chem 43:279–284
Li WY, Zheng HK, Wang JW, Zhang LL, Han HM, Wu MX (2017) Thiolate/disulfide organic redox couples for efficient organic dye-sensitized solar cells. Appl Phys A 123:541
Liu G, Li X, Wang H, Rong Y, Ku Z, Xu M, Liu L, Hu M, Yang Y, Han H (2013) An efficient thiolate/disulfide redox couple based dye-sensitized solar cell with a graphene modified mesoscopic carbon counter electrode. Carbon 53:11–18
El-Zohry AM, Cong J, Karlsson M, Kloo L, Zietz B (2016) Ferrocene as a rapid charge regenerator in dye-sensitized solar cells. Dyes Pigments 132:360–368
Flores-Leonar MM, Moreno-Esparza R, Ugalde-Saldívar VM, Amador-Bedolla C (2017) Further insights in DFT calculations of redox potential for iron complexes: the ferrocenium/ferrocene system. Comput Theor Chem 1099:167–173
Flores-Díaz N, Soto-Navarro A, Freitag M, Lamoureux G, Pineda LW (2018) Neutral organic redox pairs based on sterically hindered hydroquinone/benzoquinone derivatives for dye-sensitized solar cells. Sol Energy 167:76–83
Yang W, Söderberg M, Eriksson AIK, Boschloo G (2015) Efficient aqueous dye-sensitized solar cell electrolytes based on a TEMPO/TEMPO + redox couple. RSC Adv 5:26706–26709
Zhang Z, Chen P, Murakami TN, Zakeeruddin SM, Grätzel M (2008) The 2,2,6,6-tetramethyl-1-piperidinyloxy radical: an efficient, iodine- free redox mediator for dye-sensitized solar cells. Adv Funct Mater 18:341–346
Kato F, Naoki H, Takaya M, Chie O, Kenichi O, Hiroyuki N (2010) Nitroxide radicals for highly efficient redox mediation in dye-sensitized solar cells. Chem Lett 39:464–465
Gryn’ova G, Barakat JM, Blinco JP, Bottle SE, Coote ML (2012) Computational design of cyclic nitroxides as efficient redox mediators for dye-sensitized solar cells. Chem Eur J 18(24):7582–7593
Yonekuta Y, Oyaizu K, Nishide H (2007) Structural implication of oxoammonium cations for reversible organic one-electron redox reaction to nitroxide radicals. Chem Lett 36:866–867
Kusama H, Sayama K (2016) Comparative study on the interactions of TEMPO and iodine with organic dyes in dye-sensitized solar cells. J Photochem Photobiol Chem 330:95–101
Pipornpong W, Wanbayor R, Ruangpornvisuti V (2011) Adsorption CO2 on the perfect and oxygen vacancy defect surfaces of anatase TiO2 and its photocatalytic mechanism of conversion to CO. Appl Surf Sci 257(24):10322–10328
Indrakanti VP, Kubicki JD, Schobert HH (2011) Photoinduced activation of CO2 on TiO2 surface: quantum chemical modeling of CO2 adsorption on oxygen vacancies. Fuel Process Technol 92(4):805–811
Richards CE, Anderson AY, Martiniani S, Law C, O’Regan BC (2012) The mechanism of iodine reduction by TiO2 electrons and the kinetics of recombination in dye-sensitized solar cells. J Phys Chem Lett 3:1980–1984
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima M, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam MJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, (2009) Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT
Liu SH, Fu H, Cheng YM, Wu KL, Ho ST, Chi Y, Chou PT (2012) Theoretical study of N749 dyes anchoring on the (TiO2)28 surface in DSSCs and their electronic absorption properties. J Phys Chem C 116(3):16338–16345
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J Chem Phys 72:5639–5648
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Hay PJ, Wadt WR (1985) Ab Inition effective core potential for moleculare calculations—potential for the transition-metal atoms Sc to Hg. J Chem Phys 82:270–283
Dolg M, Wedig U, Stoll H, Preuss H (1987) Energy-adjusted ab initio pseudopotentials for the first row transition elements. J Chem Phys 86:866–872
Hanweel MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization and analysis platform. J Cheminform 4:17
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
O’Boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular ware functions. I. J Chem Phys 23(10):1833–1840
Guerra CF, Handgraaf J-W, Baerends EJ, Bickelhaupt FM (2004) Voronoi deformation density (VDD) charges; assesment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD methods for charge analysis. J Comput Chem 25(2):189–210
Ona OB, De Clercq O, Alcoba DR, Torre A, Lain L, Neck DV, Bultinck P (2016) Atom and bond Fukui function and matrices: a-Hirsfield-I atom in molecule approach. Chem Phys Chem 17:2881–2889
Gobal F, Arab R, Nahali M (2010) A comparative DFT study of atomic and molecular oxygen adsorption on neutral and negatively charged PdxCu3x (x = 0–3) nano-clusters. J Mol Struct THEOCHEM 959:15–21
Paramasivam M, Chitumalla RK, Jang J, Youk JH (2018) The impact of heteroatom substitution on cross-conjugation and its effect on the photovoltaic performance of DSSCs—a computational investigation of linear vs. cross-conjugated anchoring units. Phys Chem Chem Phys 20(35):22660–22673
Shokuhi RA, Jouibary YM, Foukolaei VP, Binaeian E (2016) Study on the structure and electronic property of adsorbed guanine on aluminum doped graphene: first principles calculations. Curr Appl Phys 16:527–533
Rad AS, Zareyee D, Peyravi M, Jahanshahi M (2016) Surface study of gallium and aluminum doped graphenes upon adsorption of cytosine: DFT calculations. Appl Surf Sci 390:444–451
Luttrell T, Halpegamage S, Tao J, Kramer A, Sutter E, Batzill M (2014) Why is anatase a better photocatalyst than rutile? Model studies on epitaxial TiO2 films. Sci Rep 4:4043
Asaduzzaman AM, Schreckenbach G (2011) Computational studies on the interations of I- and I3- with TiO2 cluster: implications for dye-sensitized solar cells. Theor Chem Acc 129:199–208
Ahn J, Lee KC, Kim D, Lee C, Lee S, Cho DW, Kyung S, Im C (2013) Synthesis of novel ruthenium dyes with thiophene or thienothiophene substituted terpyridyl ligands and their characterization. Mol Cryst Liq Cryst 581(1):45–51
Aghaei SM, Monshi MM, Torres I, Zeidi SMJ, Calizo I (2018) DFT study of adsorption behavior of NO, CO, NO2, and NH3 molecules on graphene-like BC3: a search for highly sensitive molecular sensor. Appl Surf Sci 427:326–333
Acknowledgements
The Ministry of Research, Technology and Higher Education of the Republic of Indonesia is acknowledged for the financial support with the contract Number 947/PKS/ITS/2018 through the PDUPT research scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kusumawati, Y., Puteri, Z.R., Ivansyah, A.L. et al. The study of nitroxide radical redox-couple and anatase surface interaction: a guide to choose the best sensitizer. Theor Chem Acc 138, 63 (2019). https://doi.org/10.1007/s00214-019-2452-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2452-z