Abstract
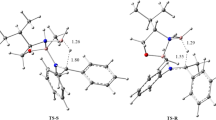
The new model for the Morita–Baylis–Hillman reaction based on the proton transfer were inquired by MP2 and DFT methods with 6-311G++(d, p) basis set combined with IEF-PCM solvent model. We focused on the reaction between acrylonitrile and benzaldehyde, catalyzed by CaO cluster and CaO modified with [Pyr][HSO4] ionic liquid. Our results indicate that in the presence of ionic liquid, the ionic liquid acts as a shuttle for the proton transfer between species in a lower energy pathway. The proton transfer step from enolate to catalyst is predicted to be the rate-limiting step for the whole process. In order to investigate the catalyst basicity, the pKa values of acrylonitrile and methyl acrylate in the presence and absence of the catalyst were measured in DMSO as a solvent. Furthermore, the proton affinities and the basicity of the CaO cluster and CaO modified with [Pyr][HSO4] ionic liquid and its components in the gas phase have been calculated at the same level of theory. Molecular electrostatic potential and valence natural atomic orbital energies in the gas phase for the catalyst have been calculated.










Similar content being viewed by others
References
Bhowmik S, Batra S (2014) Curr Org Chem 18:3078
Othman MR, Helwani Z, Fernando W (2009) Appl Organomet Chem 23:335
Kannan S (2006) Catal Surv Asia 10:117
Debecker DP, Gaigneaux EM, Busca G (2009) Chemistry 15:3920
Basavaiah D, Rao K, Reddy R (2007) J Chem Soc Rev 36:1581
Basavaiah D, Rao K, Satyanarayana A (2003) Chem Rev 103:811
Aggarwal VK, Meeru A (1999) Chem Commun 125:2311
Kataoka T, Iwama T, Kinoshita H, Surukami S, Iwwamura T, Watanabe S (1999) Synlett 125:197
Hayase T, Shibata TS, Soai K, Wakatsuki Y (1998) Chem Commun 46:1271
Rose P, Clifford A, Rayner C (2002) Chem Commun 9:968
Chandrasekhar S, Narsihmulu C, Saritha B, Sultana S (2004) Tetrahedron Lett 45:5865
Ge S-Q, Hua Y-Y, Xia M (2009) Ultrason Sonochem 16:743
Kundu M, Mukherjee S, Balu N, Padmakumar R, Bhat S (1994) Synlett 6:444
Chowdhury S, Mohan R, Scott J (2007) Tetrahedron 63:2363
Santhosh K, Samanta A (2010) J Phys Chem B 114:9195
Nagasawa Y, Oishi A, Itoh T, Muramatsu MYM, Ishibashi Y, Ito S, Miyasaki H (2009) J Phys Chem B 113:11868
Vieira R, Falvey D (2008) J Am Chem Soc 130:1552
Hunger J, Stoppa A, Buchnner R, Hefter G (2008) J Phys Chem B 112:12913
Hu Z, Margulis C (2006) J Phys Chem B 110:11025
Chowdhury P, Halder M, Sanders L, Calhoun T, Anderson J, Armstrong D, Song X, Petrich J (2004) J Phys Chem B 108:10245
Bourlinos AB, Raman K, Herrera R, Zhang Q, Archer LA, Giannelis EPA (2004) J Am Chem Soc 126:15358
Rosa JN, Afonso AM, Santos AG (2001) Tetrahedron 57:4189
Xueling M, Sanzhong L, Cheng JP (2005) J Org Chem 70:2338
Huh S, Chen H-T, Wiench JW, Pruski M, Lin VSY (2004) J Am Chem Soc 126:1010
Tanabe K, Holderich WF (1999) Appl Catal A 181:399
Huang J-W, Shi M (2003) Adv Synth Catal 345:953
Corm A, García H, Leyva A (2003) Chem Commun 63:2806
Yu C, Liu B, Hu L (2001) J Org Chem 66:5413
Yu C, Hu L (2002) J Org Chem 67:219
Stewart R (1985) The proton: appellation to organic chemistry. Academic Press, NewYork
Carrol FA (1998) Perspectives on structure and mechanism in organic chemistry. Brooks-Cole, New York
Zhao J, Zhang RY (2004) Atmos Environ 38:2177
Enami S, Mishra H, Hoffmann MR, Colussi AJ (2012) J Phys Chem A 116:6027
Kennedy RA, Mayhew CA, Thomas R, Watts P (2003) Int J Mass Spectrom 223:627
Uggerud E (1992) Mass Spectrom Rev 11:389
Head-Gordon M, Pople JA, Frisch MJ (1988) Chem Phys Lett 153:503
Head-Gordon M, Head-Gordon T (1994) Chem Phys Lett 220:122
Frisch MJ et al (2009) GAUSSIAN 09, revision B.01. Gaussian Inc, Wallingford
Zobeydi R, Setayesh SR (2018) Chem Phys 504:31
Becke A (1988) Phys Rev A 38:3098
Lee C, Yang W, Parr G (1988) Phys Rev B 37:785
Cances M, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032
Mennucci B, Tomasi J (1997) J Chem Phys 106:5151
Cossi M, Scalmani G, Rega N, Barone V (2002) J Chem Phys 117:43
Fukui K (1970) J Phys Chem 74:4161
Arnett M (1984) J Am Chem Soc 106:6759
Martin JML, Francois JP, Gijbels R (1989) J Comput Chem 10:346
Hwang SG, Jang YH, Chung DS (2005) Bull Korean Chem Soc 26:585
Topol IA, Tawa GJ, Burt SK, Rashin AA (1997) J Phys Chem A 101:10075
Richard JP (1998) Biochemistry 37:4305
Kirby A (1997) Acc Chem Res 30:290
Bartmess JE (2011). In: Mallard WG, Linstrom PJ (eds) NIST chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg, MD 20899. (http://webbook.nist.gov)
Correa JV, Jaque P, Olah J, Toro-Labbe A, Geerlings P (2009) Chem Phys Lett 470:180
Labet V, Morell C, Toro-Labbe A, Grand A (2010) Phys Chem Chem Phys 12:4142
Bode M (1991) Tetrahedron Lett 32:5611
Price K, Broadwater S, McQuade H (2005) Org Lett 7:147
Xu J (2006) J Mol Struct THEOCHEM 767:61
Acknowledgements
Our special thanks go to the Department of Chemistry and High Performance Computing Center (SHPCC) of Sharif University of Technology to provide the computational resources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zobeydi, R., Rahman Setayesh, S. Designed model for the Morita–Baylis–Hillman reaction mechanism in the presence of CaO and CaO modified with ionic liquid as a solid base catalyst: a DFT and MP2 investigation. Theor Chem Acc 137, 123 (2018). https://doi.org/10.1007/s00214-018-2306-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2306-0