Abstract
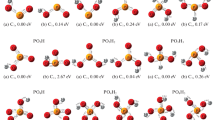
The reducibility of bulk metal oxides in which the cation is in its highest oxidation state (MgO, Sc2O3, Y2O3, TiO2, m-ZrO2, m-HfO2, CeO2, V2O5, Nb2O5, Ta2O5, WO3, CrO3, Al2O3, β-Ga2O3, SiO2, SnO2 and ZnO) has been studied by standard periodic density functional theory. We have defined and calculated descriptors able to describe and quantify semi-quantitatively the extent of reduction: electronic band gap, oxygen vacancy formation energy and electronic localization. We find that there is no single criterion for characterizing the reducibility. We discuss the advantages and limitations of each method, and we apply them to classify the materials with the PBE+U and B3LYP functionals. Typical irreducible oxides such as MgO show a large band gap, high oxygen vacancy formation energy and electronic localization of the reduction electrons forming and F-center, with a diamagnetic singlet electronic state. Reducible oxides such as TiO2 present small band gaps, small oxygen vacancy formation energy and electron localization of the reduction electrons in the cations, decreasing their oxidation state and presenting open-shell electronic states. Intermediate or ambivalent behavior is found for ZrO2, HfO2, β-Ga2O3, ZnO and SnO2.







Similar content being viewed by others
References
Helali Z, Markovits A, Minot C, Abderrabba M (2012) First row transition metal atoms adsorption on rutile TiO2(110) surface. Struct Chem 23:1309
Helali Z, Markovits A, Minot C, Abderrabba M (2014) Metal atom adsorption on a defective TiO2−x support. Chem Phys Lett 594:23–29
Bond GC, Tahir SF (1991) Vanadium oxide monolayer catalysts preparation, characterization and catalytic activity. Appl Catal 71(1):1–31
Deo G, Wachs IE, Haber J (1994) Supported vanadium oxide catalysts:’molecular structural characterization and reactivity properties. Crit Rev Surf Chem 4:141
Weckhuysen BM, Keller DE (2003) Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal Today 78(1):25–46
Summers JC, Ausen SA (1979) Interaction of cerium oxide with noble metals. J Catal 58(1):131–143
Tauster SJ, Fung SC, Baker RTK, Horsley JA (1981) Strong interactions in supported metal catalysts. Science 211(4487):1121–1125
Tauster SJ, Fung SC, Garten RL (1978) Strong metal-support interactions—group 8 noble-metals supported on TiO2. J Am Chem Soc 100(1):170–175
Sousa C, Illas F (1994) Ionic-covalent transition in titanium oxides. Phys Rev B Condens Matter 50:13974–13980
Sanderson RT (1983) Electronegativity and bond energy. J Am Chem Soc 105(8):2259–2261. doi:10.1021/ja00346a026
Hill YD, Huang Y, Ast T, Freiser BS (1997) Study of the gas-phase chemistry of Y2+ with small alkanes. Rapid Commun Mass Spectrom 11(1):148–154. doi:10.1002/(SICI)1097-0231(19970115)11:1<148:AID-RCM819>3.0.CO;2-5
Sharma TC, Sharma SC, Bhandari KS (1984) Polarographic behaviour of yttrium. Analyst 109(12):1615–1616. doi:10.1039/an9840901615
Beltrán A, Andrés J, Calatayud M, Martins JBL (2001) Theoretical study of ZnO (1 0 1 0) and Cu/ZnO (1 0 1 0) surfaces. Chem Phys Lett 338(4–6):224–230
Calatayud M, Markovits A, Menetrey M, Mguig B, Minot C (2003) Adsorption on perfect and reduced surfaces of metal oxides. Catal Today 85(2–4):125–143
Calatayud M, Markovits A, Minot C (2004) Electron-count control on adsorption upon reducible and irreducible clean metal-oxide surfaces. Catal Today 89(3):269–278
Calatayud M, Markovits A, Minot C (2008) Periodic DFT studies on adsorption and reactivity on metal and metal oxide surfaces. In: Basiuk VA, Ugliengo P (eds) Quantum chemical calculations of surfaces and interfaces of materials, Chapter 11. American Scientific Publisher, Cambridge, pp 183–210
Ganduglia-Pirovano MV, Hofmann A, Sauer J (2007) Oxygen vacancies in transition metal and rare earth oxides: current state of understanding and remaining challenges. Surf Sci Rep 62(6):219–270
Menetrey M, Markovits A, Minot C (2003) Reactivity of a reduced metal oxide surface: hydrogen, water and carbon monoxide adsorption on oxygen defective rutile TiO2(1 1 0). Surf Sci 524(1–3):49–62
Calatayud M, Minot C (2006) Reactivity of the V2O5–TiO2-anatase catalyst: role of the oxygen sites. Top Catal 41(1–4):17–26. doi:10.1007/s11244-006-0090-x
Calatayud M, Minot C (2009) Is there a nanosize for the activity of TiO2 compounds? J Phys Chem C 113(28):12186–12194. doi:10.1021/jp901465q
Calatayud M, Tielens F, De Proft F (2008) Reactivity of gas-phase, crystal and supported V2O5 systems studied using density functional theory based reactivity indices. Chem Phys Lett 456(1–3):59–63. doi:10.1016/j.cplett.2008.03.007
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50(24):17953–17979
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59(3):1758–1775
Calatayud M (2017). https://sites.google.com/site/calatayudantonino/k/oxides
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186
Kresse G, Furthmüller J (1996) Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6(1):15–50
Kresse G, Hafner J (1993) Ab initio molecular dynamics for open-shell transition metals. Phys Rev B 48(17):13115–13118
Kresse G, Hafner J (1994) Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J Phys Condens Matter 6(40):8245
Momma K, Izumi F (2008) VESTA: a three-dimensional visualization system for electronic and structural analysis. J Appl Crystallogr 41(3):653–658. doi:10.1107/S0021889808012016
Morgan BJ, Watson GW (2007) A DFT+U description of oxygen vacancies at the TiO2 rutile (110) surface. Surf Sci 601(21):5034–5041
Morgan BJ, Watson GW (2009) A density functional theory+ U study of oxygen vacancy formation at the (110), (100), (101), and (001) surfaces of rutile TiO2. J Phys Chem C 113(17):7322–7328. doi:10.1021/jp811288n
Jedidi A, Markovits A, Minot C, Bouzriba S, Abderraba M (2010) Modeling localized photoinduced electrons in rutile-TiO2 using periodic DFT+U methodology. Langmuir 26(21):16232–16238. doi:10.1021/la101359m
Morgan BJ, Watson GW (2010) Intrinsic n-type defect formation in TiO2: a comparison of rutile and anatase from GGA plus U calculations. J Phys Chem C 114(5):2321–2328. doi:10.1021/jp9088047
Scanlon DO, Walsh A, Morgan BJ, Watson GW (2008) An ab initio study of reduction of V2O5 through the formation of oxygen vacancies and Li intercalation. J Phys Chem C 112(26):9903–9911. doi:10.1021/jp711334f
Laubach S, Schmidt PC, Thissen A, Fernandez-Madrigal FJ, Wu QH, Jaegermann W, Klemm M, Horn S (2007) Theoretical and experimental determination of the electronic structure of V2O5, reduced V2O5−x and sodium intercalated NaV2O5. Phys Chem Chem Phys 9(20):2564–2576. doi:10.1039/b612489e
Fabris S, de Gironcoli S, Baroni S, Vicario G, Balducci G (2005) Taming multiple valency with density functionals: a case study of defective ceria. Phys Rev B 71(4):041102
Cococcioni M, de Gironcoli S (2005) Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys Rev B 71(3):035105
Fabris S, de Gironcoli S, Baroni S, Vicario G, Balducci G (2005) Reply to Comment on ‘Taming multiple valency with density functionals: A case study of defective ceria’. Phys Rev B 72(23):237102
Kresse G, Blaha P, Da Silva JLF, Ganduglia-Pirovano MV (2005) Comment on “Taming multiple valency with density functionals: A case study of defective ceria”. Phys Rev B 72(23):237101
Fabris S, Vicario G, Balducci G, de Gironcoli S, Baroni S (2005) Electronic and atomistic structures of clean and reduced ceria surfaces. J Phys Chem B 109(48):22860–22867. doi:10.1021/jp0511698
Nolan M, Grigoleit S, Sayle DC, Parker SC, Watson GW (2005) Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria. Surf Sci 576:217–229
Jiang Y, Adams JB, van Schilfgaarde M (2005) Density-functional calculation of CeO2 surfaces and prediction of effects of oxygen partial pressure and temperature on stabilities. J Chem Phys 123(6):064701–064709
Jiang Y, Adams JB, van Schilfgaarde M, Sharma R, Crozier PA (2005) Theoretical study of environmental dependence of oxygen vacancy formation in CeO2. Appl Phys Lett 87(14):141917. doi:10.1063/1.2084324
Loschen C, Carrasco J, Neyman KM, Illas F (2007) First-principles LDA+U and GGA+U study of cerium oxides: dependence on the effective U parameter. Phys Rev B 75(3):035115
Ivanov MV, Perevalov TV, Aliev VS, Gritsenko VA, Kaichev VV (2011) Electronic structure of Δ-Ta2O5 with oxygen vacancy: ab initio calculations and comparison with experiment. J Appl Phys 110(2):024115
Kaczkowski J (2012) Electronic structure of some wurtzite semiconductors: hybrid functionals versus ab initio many body calculations. Acta Phys Pol A 121:1142
Lany S (2008) Semiconductor thermochemistry in density functional calculations. Phys Rev B 78(24):245207
Erhart P, Albe K, Klein A (2006) First-principles study of intrinsic point defects in ZnO: role of band structure, volume relaxation, and finite-size effects. Phys Rev B 73(20):205203
Garza AJ, Scuseria GE (2016) Predicting band gaps with hybrid density functionals. J Phys Chem Lett 7(20):4165–4170. doi:10.1021/acs.jpclett.6b01807
Heyd J, Scuseria GE, Ernzerhof M (2003) Hybrid functionals based on a screened Coulomb potential. J Chem Phys 118(18):8207–8215
Heyd J, Scuseria GE, Ernzerhof M (2006) Erratum: “Hybrid functionals based on a screened Coulomb potential” [J. Chem. Phys. 118, 8207 (2003)]. J Chem Phys 124(21):219906
Henderson TM, Paier J, Scuseria GE (2011) Accurate treatment of solids with the HSE screened hybrid. Phys Status Solidi (b) 248(4):767–774. doi:10.1002/pssb.201046303
Lucero MJ, Henderson TM, Scuseria GE (2012) Improved semiconductor lattice parameters and band gaps from a middle-range screened hybrid exchange functional. J Phys Condens Matter 24(14):145504
Brix P, Herzberg G (1953) The dissociation energy of oxygen. J Chem Phys 21(12):2240
Gray HB (1994) Chemical bonds: an introduction to atomic and molecular structure. University Science Books, Mill Valley, California
Hammer B, Hansen LB, Norskov JK (1999) Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys Rev B 59(11):7413–7421
Wang L, Maxisch T, Ceder G (2006) Oxidation energies of transition metal oxides within the GGA+U framework. Phys Rev B 73(19):195107
Scienomics (2004–2015) Introduction to MAPS. Paris
Finazzi E, Di Valentin C, Pacchioni G, Selloni A (2008) Excess electron states in reduced bulk anatase TiO2: comparison of standard GGA, GGA plus U, and hybrid DFT calculations. J Chem Phys 129(15):154113. doi:10.1063/1.2996362
Janotti A, Varley JB, Rinke P, Umezawa N, Kresse G, Van de Walle CG (2010) Hybrid functional studies of the oxygen vacancy in TiO2. Phys Rev B 81(8):085212. doi:10.1103/PhysRevB.81.085212
Angenault J (2001) Symetrie et structure. Vuibert, Paris
Hannic F, Hartmanova M (1984) Real structure of undopped Y2O3 single cristal. Acta Cryst B 40:76–82
Garcia JC, Scolfaro LMR, Lino AT, Freire VN, Farias GA, Silva CC, Alves HWL, Rodrigues SCP, da Silva Jr EF (2006) Structural, electronic, and optical properties of ZrO2 from ab initio calculations. J Appl Phys 100(10):104103
Hann RE, Suitch PR, Pentecost JL (1985) Monoclinic crystal structures of ZrO2 and HfO2 refined from X-ray powder diffraction data. J Am Ceram Soc 68(10):C-285–C-286. doi:10.1111/j.1151-2916.1985.tb11534.x
Gerward L, Olsen JS, Petit L, Vaitheeswaran G, Svane KVA (2005) Bulk modulus of CeO2 and PrO2—an experimental and theoretical study. J Alloys Compd 400:56
Enjalbert R, Galy J (1986) A refinement of the structure of V2O5. Acta Cryst C 42:1467
Kato K (1976) Structure refinement of H-Nb2O5. Acta Cryst B32:764–767
Fukumoto A, Miwa K (1997) Prediction of hexagonal Ta2O5 structure by first-principles calculations. Phys Rev B Condens Matter 55:11155–11160
Jalili H (2008) Materials physics of half-metallic magnetic oxide films by pulsed laser deposition: controlling the crystal structure and near-surface properties of Sr2FeMoO6 and CrO2 films. University of Waterloo, Waterloo
Sawai Y, Hazu K, Chichibu SF (2010) Surface stoichiometry and activity control for atomically smooth low dislocation density ZnO and pseudomorphic MgZnO epitaxy on a Zn-polar ZnO substrate by the helicon-wave-excited-plasma sputtering epitaxy method. J Appl Phys 108(6):063541. doi:10.1063/1.3485600
Madelung O, Rössler U, Schulz M (2000) WO3: transport properties, monoclinic phase. In: Madelung O, Rössler U, Schulz M (eds) Non-tetrahedrally bonded binary compounds II. Springer, Berlin, pp 1–8. doi:10.1007/b71139
Lassner EW-DS (1999) Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Kluwer Academic, New York
Saki K, Kenji T, Nobuo I (2008) Structural evolution of corundum at high temperatures. Jpn J Appl Phys 47(1S):616
Geller S (1960) Crystal Structure of β-Ga2O3. J Chem Phys 33(3):676–684. doi:10.1063/1.1731237
Haiying He RO, Blanco Miguel A, Pandey Ravindra (2006) First-principles study of the structural, electronic, and optical properties of Ga2O3 in its monoclinic and hexagonal phases. Phys Rev B 74:195123. doi:10.1103/PhysRevB.74.195123
Lager GA, Jorgensen JD, Rotella FJ (1982) Crystal structure and thermal expansion of α-quartz SiO2 at low temperatures. J Appl Phys 53(10):6751–6756. doi:10.1063/1.330062
Haines J, Léger JM (1997) X-ray diffraction study of the phase transitions and structural evolution of tin dioxide at high pressure: ffRelationships between structure types and implications for other rutile-type dioxides. Phys Rev B 55(17):11144–11154
Postnikov AV, Entel P, Ordejon P (2002) SnO2: bulk and surface simulations by an ab initio numerical local orbitals method. Phase Transit Multinatl J 75(1–2):143–149
Kisi EH, Elcombe MM (1989) U parameters for the Wurtzite structure of ZnS and ZnO using powder neutron diffraction. Acta Cristallogr C 45:1867
Orita M, Ohta H, Hirano M, Hosono H (2000) Deep-ultraviolet transparent conductive beta-Ga2O3 thin films. Appl Phys Lett 77(25):4166–4168
Deml AM, Holder AM, O’Hayre RP, Musgrave CB, Stevanović V (2015) Intrinsic material properties dictating oxygen vacancy formation energetics in metal oxides. J Phys Chem Lett 6(10):1948–1953. doi:10.1021/acs.jpclett.5b00710
Di Valentin C, Pacchioni G (2014) Spectroscopic properties of doped and defective semiconducting oxides from hybrid density functional calculations. Acc Chem Res 47(11):3233–3241. doi:10.1021/ar4002944
Jun C, Lin L, Lu T, Yong L (1999) Electronic structure of F, F+-center in MgO. Eur Phys J B 9(4):593–598
Zhukovskii YF, Kotomin EA, Evarestov RA, Ellis DE (2007) Periodic models in quantum chemical simulations of F centers in crystalline metal oxides. Int J Quantum Chem 107(14):2956–2985. doi:10.1002/qua.21483
Kantorovich LN, Holender JM, Gillan MJ (1995) The energetics and electronic structure of defective and irregular surfaces on MgO. Surf Sci 343(3):221–239
Klein BM, Pickett WE, Boyer LL (1987) Theory of F-centers in the alkaline earth oxides MgO and CaO. Phys Rev B 35(11):5802–5815
Kasap SO, Capper P (2006) Springer handbook of electronic and photonic materials. Springer, Berlin. doi:10.1007/978-0-387-29185-7
Morkoç H, Özgür Ü (2009) Zinc oxide: fundamentals, materials and device technology. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Leiter F, Alves H, Pfisterer D, Romanov NG, Hofmann DM, Meyer BK (2003) Oxygen vacancies in ZnO. Physica B 340–342:201–204
Smith JM, Vehse WE (1970) ESR of electron irradiated ZnO confirmation of the F+ center. Phys Lett A 31(3):147–148
Soriano V, Galland D (1976) Photosensitivity of the EPR spectrum of the F+ center in ZnO. Phys Status Solidi (b) 77(2):739–743
van Craeynest F, Maenhout-Van Der Vorst W, Dekeyser W (1965) Interpretation of the yellow colour of heat treated ZnO powder. Phys Status Solidi (b) 8(3):841–846
Wei WF (1977) F+ center in ZnO. Phys Rev B 15(4):2250–2253
Himmel H-J, Manceron L, Downs AJ, Pullumbi P (2002) Formation and characterization of the gallium and indium subhydride molecules Ga2H2 and In2H2: a matrix isolation study. J Am Chem Soc 124(16):4448–4457
Jegier JA, Gladfelter WL (2000) The use of aluminum and gallium hydrides in materials science. Coord Chem Rev 206–207:631–650
Acknowledgements
Authors acknowledge B. Diawara for the Modelview program and Scienomics for the MAPS courtesy license. This work was performed using HPC resources from GENCI- CINES/IDRIS (Grants 2013- x2013082131, 2014- x2014082131, 2015- x2015082131, 2016- x2016082131, 2017- x2012082131), the CCRE-DSI of Université P. M. Curie and KAUST HPC supercomputer Shaheen under project k1087. This work has been carried out in the frame of the COST action CM1104 Reducible metal oxides.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles derived from the 10th Congress on Electronic Structure: Principles and Applications (ESPA-2016).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Helali, Z., Jedidi, A., Syzgantseva, O.A. et al. Scaling reducibility of metal oxides. Theor Chem Acc 136, 100 (2017). https://doi.org/10.1007/s00214-017-2130-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-017-2130-y