Abstract
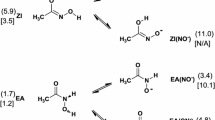
The specificities of application of the supermolecule method to the calculation of mechanisms of reactions in a protonodonor medium are considered using as an example the reaction of methylamine with ethylene carbonate in methanol. The energies of non-interacting solvated reactants have been used as the reference levels for calculation of relative energies. The problems that result from using the energies of the pre-reaction complexes or the sums of the energies of infinitely separated reactants as the reference levels are noted. The optimal number of the solvent molecules necessary to comprehensively model the reaction under consideration is found. Two possibilities are established for the stabilization of transition states and lowering of activation barriers due to the formation of cycles involving hydrogen bonds. The formation of eight-membered proton-transfer cycles and eight- or ten-membered stabilization cycles is preferable for the given reaction. The lower calculated magnitude of the barrier of the reaction proceeding by concert mechanism in methanol is 15.1 kcal mole−1. The reaction mechanism specificities associated with the formation of zwitterionic intermediates are discussed.









Similar content being viewed by others
References
Oie T, Loew GH, Burt SK, Binkley JS, McElroy RD (1982) Quantum chemical studies of a model for peptide bond formation: formation of formamide and water from ammonia and formic acid. J Am Chem Soc 104:6169–6174
Williams IH (1987) Theoretical modeling of specific solvation effects upon carbonyl addition. J Am Chem Soc 109:6299–6307
Wang L, Zipse H (1996) Bifunctional catalysis of ester aminolysis—a computational and experimental study. Liebigs Ann 1996:1501–1509
Zipse H, Wang L, Houk KN (1996) Polyether catalysis of ester aminolysis—a computational and experimental study. Liebigs Ann 1996:1511–1522
Adalstensson H, Bruice TC (1998) What is the mechanism of catalysis of ester aminolysis by weak amine bases? Comparison of experimental studies and theoretical investigation of the aminolysis of substituted phenyl esters of quinoline-6-and-8-carboxylic acids. J Am Chem Soc 120:3440–3447
Díaz N, Suárez D, Sordo TL (1999) Ammonolysis and aminolysis of β-lactams: a theoretical study. Chem Eur J 5:1045–1054
Díaz N, Suárez D, Sordo TL (1999) NH3-assisted ammonolysis of β-lactams: a theoretical study. J Org Chem 64:3281–3289
Díaz N, Suárez D, Sordo TL (1999) Importance of a synperiplanar stepwise mechanism through neutral intermediates in the aminolysis of monocyclic β-lactams: a theoretical analysis. J Org Chem 64:9144–9152
O’Hair RAJ, Androutsopoulos NK (2000) Can transacylation reactions occur via SN2 pathways in the gas phase? Insights via ion-molecule reactions of N-acylpyridinium ions and ab initio calculations. Org Lett 2:2567–2570
Kim CK, Li HG, Lee HW, Sohn CK, Chun YI, Lee I (2000) Ab initio study of the X− + RCOY displacement reactions with R=H, CH3 and X, Y=Cl. Br J Phys Chem A 104:4069–4076
Díaz N, Suárez D, Sordo TL (2000) Theoretical study of the water-assisted aminolysis of β-lactams: implications for the reaction between human serum albumin and penicillins. J Am Chem Soc 122:6710–6719
Singleton DA, Merrigan SR (2000) Resolution of conflicting mechanistic observations in ester aminolysis. A warning on the qualitative prediction of isotope effects for reactive intermediates. J Am Chem Soc 122:11035–11036
Chalmet S, Harb W, Ruiz-Lopez MF (2001) Computer simulation of amide bond formation in aqueous solution. J Phys Chem A 105:11574–11581
Díaz N, Suárez D, Sordo TL, Merz KM Jr (2001) A theoretical study of the aminolysis reaction of lysine 199 of human serum albumin with benzylpenicillin: consequences for immunochemistry of penicillins. J Am Chem Soc 123:7574–7583
Díaz N, Suárez D, Sordo TL (2001) Theoretical study of amine-assisted aminolysis of penicillins—the kinetic role of the carboxylate group. Eur J Org Chem 2001:793–801
Díaz N, Suárez D, Sordo TL (2002) Theoretical study of ammonolysis of monobactams: kinetic role of the N-sulfonate group. Helv Chim Acta 85:206–223
Ilieva S, Galabov B, Musaev DG, Morokuma K (2003) Computational study of the aminolysis of 2-benzoxazolinone. J Org Chem 68:3406–3412
Díaz N, Suárez D, Sordo TL, Méndez R, Villacorta JMA (2003) Combined theoretical and experimental research project into the aminolysis of β-lactam antibiotics: the importance of bifunctional catalysis. Eur J Org Chem 2003:4161–4172
Ilieva S, Galabov B, Musaev DG, Morokuma K, Schaefer HF (2003) Computational study of the aminolysis of esters. the reaction of methylformate with ammonia. J Org Chem 68:1496–1502
Ilieva S, Atanasov Y, Kalcheva V, Galabov B (2003) Computational study of the general base catalysed aminolysis of 2-benzoxazolinone. J Mol Struct Theochem 633:49–55
Galabov B, Atanasov Y, Ilieva S, Schaefer HF III (2005) Mechanism of the aminolysis of methyl benzoate: a computational study. J Phys Chem A 109:11470–11474
Sung DD, Koo IS, Yang K, Lee I (2006) DFT studies on the structure and stability of zwitterionic tetrahedral intermediate in the aminolysis of esters. Chem Phys Lett 426:280–284
Jin L, Wu Y, Xue Y, Guo Y, Xie DQ, Yan GS (2006) Theoretical studies on the aminolysis of phenyl formate. mechanism and solvent effect. Acta Chim Sin 64:873–878
Xia X, Zhang C, Xue Y, Kim CK, Yan G (2008) DFT study and Monte Carlo simulation on the aminolysis of XC(O)OCH3 (X=NH2, H, and CF3) with monomeric and dimeric ammonias. J Chem Theory Comput 4:1643–1653
Petrova T, Okovytyy S, Gorb L, Leszczynski J (2008) Computational study of the aminolysis of anhydrides: effect of the catalysis to the reaction of succinic anhydride with methylamine in gas phase and nonpolar solution. J Phys Chem A 112:5224–5235
Ilieva S, Atanasov Y, Galabov B (2008) Mechanism of the aminolysis of phenyl acetate: a computational study. Bulg Chem Commun 40:401–408
Han IS, Kim CK, Sohn CK, Ma EK, Lee HW, Kim CK (2011) Comparative studies on the reactions of acetyl and thioacetyl halides with NH3 in the gas phase and in aqueous solution: a theoretical study. J Phys Chem A 115:1364–1370
Zabalov MV, Tiger RP, Berlin AA (2011) Reaction of cyclocarbonates with amines as an alternative route to polyurethanes: a quantum-chemical study of reaction mechanism. Dokl Phys Chem (Engl Transl) 441:355–360
Zabalov MV, Tiger RP, Berlin AA (2012) Mechanism of urethane formation from cyclocarbonates and amines: a quantum chemical study. Russ Chem Bull (Int Ed) 61:518–527
Liu XQ, Jin L, Kim CK, Xue Y (2012) Role of bifunctional catalyst 2-pyridone in the aminolysis of p-nitrophenyl acetate with n-butylamine: a computational study. J Mol Catal A Chem 355:102–112
Kim CK, Han IS, Sohn CK, Yu YH, Su Z, Kim CK (2012) Comparative studies on the reactions of carbamyl and thiocarbamyl halides with NH3 in the gas phase and in aqueous solution: a theoretical study. Bull Korean Chem Soc 33:1955–1961
Rao HB, Wang YY, Zeng XY, Xue Y, Li ZR (2013) Theoretical study on the aminolysis of p-substituted phenyl acetates with dimeric ammonia in vacuo and acetonitrile. Comput Theor Chem 1008:8–14
Zabalov MV, Levina MA, Krasheninnikov VG, Tiger RP (2014) Bifunctional catalysis by acetic acid in the urethane formation from cyclocarbonates and amines: quantum chemical and kinetic study. Russ Chem Bull (Int Ed) 63:1740–1752
Chen R, Luo X, Liang G (2015) Computational study on the aminolysis mechanism of 4,4-dimethyl-2-vinyl-2-oxazolin-5-one with methylamine. Comput Theor Chem 1073:84–93
Chen R, Luo X, Liang G (2015) Theoretical studies on the aminolysis mechanism of propylene carbonate with ammonia. Theor Chem Acc 134:32
Yuen A, Bossion A, Gómez-Bengoa E, Ruipérez F, Isik M, Hedrick JL, Mecerreyes D, Yang YY, Sardon H (2016) Room temperature synthesis of non-isocyanate polyurethanes (NIPUs) using highly reactive n-substituted 8-membered cyclic carbonates. Polym Chem 7:2105–2111
Bossion A, Jones GO, Taton D, Mecerreyes D, Hedrick JL, Ong ZY, Yang YY, Sardon H (2017) Non-isocyanate polyurethane soft nanoparticles obtained by surfactant-assisted interfacial polymerization. Langmuir 33:1959–1968
Raushel FM, Villafranca JJ (1980) Theoretical models for transition-state structure and catalysis in carbonyl addition. J Am Chem Soc 102:6619–6621
Williams IH, Maggiora GM, Schowen RL (1980) Theoretical models for mechanism and catalysis in carbonyl addition. J Am Chem Soc 102:7831–7839
Williams IH, Spangler D, Femec DA, Maggiora GM, Schowen RL (1983) Theoretical models for solvation and catalysis in carbonyl addition. J Am Chem Soc 105:31–40
Antonczak S, Ruiz-Lopez MF, Rivail JL (1994) Ab initio analysis of water-assisted reaction mechanisms in amide hydrolysis. J Am Chem Soc 116:3912–3921
Wolfe S, Kim CK, Yang K, Weinberg N, Shi Z (1995) Hydration of the carbonyl group. a theoretical study of the cooperative mechanism. J Am Chem Soc 117:4240–4260
Kallies B, Mitzner R (1998) Models of water-assisted hydrolyses of methyl formate, formamide, and urea from combined DFT–SCRF calculations. J Mol Model 4:183–196
Schmeer G, Sturm PA (1999) Quantum chemical approach to the water assisted neutral hydrolysis of ethyl acetate and its derivatives. Phys Chem Chem Phys 1:1025–1030
Hori K, Hashitani Y, Kaku Y, Ohkubo K (1999) Theoretical study on oxygen exchange accompanying alkaline hydrolysis of esters and amides. The role of water for the exchange reaction. J Mol Struct Theochem 461–462:589–596
Fox JM, Dmitrenko O, Liao L, Bach RD (2004) Computational studies of nucleophilic substitution at carbonyl carbon: the SN2 mechanism versus the tetrahedral intermediate in organic synthesis. J Org Chem 69:7317–7328
Yamabe S, Tsuchida N, Hayashida Y (2005) Reaction paths of the water-assisted neutral hydrolysis of ethyl acetate. J Phys Chem A 109:7216–7224
Simón L, Goodman JM (2007) The mechanism of TBD-catalyzed ring-opening polymerization of cyclic esters. J Org Chem 72:9656–9662
Buis N, French SA, Ruggiero GD, Stengel B, Tulloch AAD, Williams IH (2007) computational investigation of mechanisms for ring-opening polymerization of ε-caprolactone: evidence for bifunctional catalysis by alcohols. J Chem Theory Comput 3:146–155
Almerindo GI, Pliego JR Jr (2007) Ab initio investigation of the kinetics and mechanism of the neutral hydrolysis of formamide in aqueous solution. J Braz Chem Soc 18:696–702
Hori K, Ikenaga Y, Arata K, Takahashi T, Kasai K, Noguchi Y, Sumimoto M, Yamamoto H (2007) Theoretical study on the reaction mechanism for the hydrolysis of esters and amides under acidic conditions. Tetrahedron 63:1264–1269
Tsuchida N, Satou H, Yamabe S (2007) Reaction paths of the water-assisted solvolysis of N, N-dimethylformamide. J Phys Chem A 111:6296–6303
Bonduelle C, Martín-Vaca B, Cossío FP, Bourissou D (2008) Monomer versus alcohol activation in the 4-dimethylaminopyridine-catalyzed ring-opening polymerization of lactide and lactic O-carboxylic anhydride. Chem Eur J 14:5304–5312
Zeng Y, Xue Y, Yan G (2008) Theoretical study of the acid-promoted hydrolysis of oxazolin-5-one: a microhydration model. J Phys Chem B 112:10659–10667
Shi Z, Hsieh Y, Weinberg N, Wolfea S (2009) The neutral hydrolysis of methyl acetate—part 2. Is there a tetrahedral intermediate? Can J Chem 87:544–555
Limpanuparb T, Panyain K, Tantirungrotechai Y (2010) A DFT investigation of methanolysis and hydrolysis of triacetin. J Mol Struct Theochem 955:23–32
Yamabe S, Fukuda T, Ishii M (2011) Role of hydrogen bonds in acid-catalyzed hydrolyses of esters. Theor Chem Acc 130:429–438
Karaman R (2011) Analyzing the efficiency in intramolecular amide hydrolysis of Kirby’s N-alkylmaleamic acids—a computational approach. Comput Theor Chem 974:133–142
Zaramello L, Kuhnen CA, Dall’Oglio EL, de Sousa PT Jr (2012) DFT study of gas phase acid-catalyzed ethanolysis of butyric acid triglyceride. Fuel 94:473–479
da Silva ACH, da Silva SC, Dall’Oglio EL, de Sousa PT Jr, Kuhnen CA (2013) The alkaline-catalyzed transesterification of monoglycerides of butyric and pentylic acids: gas-phase and solvent effects. Fuel 104:379–385
da Silva ACH, Kuhnen CA, da Silva SC, Dall’Oglio EL, de Sousa PT Jr (2013) DFT study of alkaline-catalyzed methanolysis of pentylic acid triglyceride: gas phase and solvent effects. Fuel 107:387–393
da Silva PL, Guimarães L, Pliego JR Jr (2013) Revisiting the mechanism of neutral hydrolysis of esters: water autoionization mechanisms with acid or base initiation pathways. J Phys Chem B 117:6487–6497
Gómez-Bombarelli R, Calle E, Casado J (2013) Mechanisms of lactone hydrolysis in neutral and alkaline conditions. J Org Chem 78:6868–6879
Silva ACH, da Dall’Oglio EL, de Sousa Jr PT, da Silva SC, Kuhnen CA (2014) DFT study of the acid-catalyzed ethanolysis of butyric acid monoglyceride: solvent effects. Fuel 119:1–5
Pratt RC, Lohmeijer BGG, Long DA, Lundberg PNP, Dove AP, Li H, Wade CG, Waymouth RM, Hedrick JL (2006) Exploration, optimization, and application of supramolecular thiourea-amine catalysts for the synthesis of lactide (co)polymers. Macromolecules 39:7863–7871
Pratt RC, Lohmeijer BGG, Long DA, Waymouth RM, Hedrick JL (2006) Triazabicyclodecene: a simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J Am Chem Soc 128:4556–4557
Kiesewetter MK, Scholten MD, Kirn N, Weber RL, Hedrick JL, Waymouth RM (2009) Cyclic guanidine organic catalysts: what is magic about triazabicyclodecene? J Org Chem 74:9490–9496
Coady DJ, Fukushima K, Horn HW, Rice JE, Hedrick JL (2011) Catalytic insights into acid/base conjugates: highly selective bifunctional catalysts for the ring-opening polymerization of lactide. Chem Comm 47:3105–3107
Lambeth RH, Henderson TJ (2013) Organocatalytic synthesis of (poly)hydroxyurethanes from cyclic carbonates and amines. Polymer 54:5568–5573
Kazakov OI, Datta PP, Isajani M, Kiesewetter ET, Kiesewetter MK (2014) Cooperative hydrogen-bond pairing in organocatalytic ring-opening polymerization. Macromolecules 47:7463–7468
Eisenreich F, Viehmann P, Müller F, Hecht S (2015) Electronic activity tuning of acyclic guanidines for lactide polymerization. Macromolecules 48:8729–8732
Kazakov OI, Kiesewetter MK (2015) Cocatalyst binding effects in organocatalytic ring-opening polymerization of L-lactide. Macromolecules 48:6121–6126
Lombardo VM, Dhulst EA, Leitsch EK, Wilmot N, Heath WH, Gies AP, Miller MD, Torkelson JM, Scheidt KA (2015) Cooperative catalysis of cyclic carbonate ring opening: application towards non-isocyanate polyurethane materials. Eur J Org Chem 2015:2791–2795
Kaoukabi A, Guillen F, Qayouh H, Bouyahya A, Balieu S, Belachemi L, Gouhier G, Lahcini M (2015) the use of ionic liquids as an organocatalyst for controlled ring-opening polymerization of ε–caprolactone. Ind Crops Prod 72:16–23
Li X, Zhang Q, Li Z, Wang X, Liu J, Cui S, Xu S, Zhao C, Chen C, Guo K (2016) Thiourea binding with carboxylic acid promoted cationic ring-opening polymerization. Polymer 84:293–303
Lamarzelle O, Durand PL, Wirotius AL, Chollet G, Grau E, Cramail H (2016) Activated lipidic cyclic carbonates for non-isocyanate polyurethane synthesis. Polym Chem 7:1439–1451
Zabalov MV, Tiger RP (2016) The supermolecule method, as applied to studies of liquid-phase reaction mechanisms taking cyclocarbonate aminolysis in dioxane as an example: specific features. Russ Chem Bull (Int Ed) 65:631–639
Levina MA, Krasheninnikov VG, Zabalov MV, Tiger RP (2014) nonisocyanate polyurethanes from amines and cyclic carbonates: kinetics and mechanism of a model reaction. Polym Sci Ser B (Engl Transl) 56:139–147
Yang W, Drueckhammer DG (2000) Computational studies of the aminolysis of oxoesters and thioesters in aqueous solution. Org Lett 2:4133–4136
Sabot C, Kumar KA, Meunier S, Mioskowski C (2007) A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) under solvent-free conditions. Tetrah Lett 48:3863–3866
Sung DD, Koo IS, Yang K, Lee I (2006) Effects of atom pairs O and S on the stability of zwitterionic tetrahedral intermediate: a theoretical study. Chem Phys Lett 432:426–430
Venkatasubban KS, Bush M, Ross E, Schultz M, Garza O (1998) Transition state structure for the water-catalyzed hydrolysis of p-nitrophenyl trifluoroacetate in acetonitrile. J Org Chem 63:6115–6118
Frasson CML, Brandão TAS, Zucco C, Nome F (2006) Solvent effect and proton inventory in the hydrolysis of p-methylphenyl trichloroacetate. J Phys Org Chem 19:143–147
Um IH, Kim MY, Bae AR, Dust JM, Buncel E (2015) Evidence for a catalytic six-membered cyclic transition state in aminolysis of 4-nitrophenyl 3,5-dinitrobenzoate in acetonitrile: comparative brønsted-type plot, entropy of activation, and deuterium kinetic isotope effects. J Org Chem 80:217–222
Zhang L, Xie D, Xu D, Guo H (2007) Supermolecule density functional calculations suggest a key role for solvent in alkaline hydrolysis of p-nitrophenyl phosphate. Chem Comm 1638–1640. doi:10.1039/b617946k
Nohra B, Candy L, Blanco JF, Guerin C, Raoul Y, Moolaungui Z (2013) From petrochemical polyurethanes to biobased polyhydroxyurethanes. Macromolecules 46:3771–3792
Figovsky O, Shapovalov L, Leykin A, Birukova O, Potashnikova R (2013) Recent advances in the development of non-isocyanate polyurethanes based on cyclic carbonates. PU Mag Int 10:256–263
Maisonneuve L, Lamarzelle O, Rix E, Grau E, Cramail H (2015) Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chem Rev 115:12407–12439
Perdew JP, Burke K, Ernzerhoff M (1996) generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Ernzerhoff M, Scuseria GE (1999) Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J Chem Phys 110:5029–5036
Laikov DN (1997) Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem Phys Lett 281:151–156
Laikov DN, Ustynuk YA (2005) PRIRODA04: a quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ Chem Bull (Int Ed) 54:820–826
Glasstone S, Laidler KJ, Eyring H (1941) The theory of rate processes. The kinetics of chemical reactions, viscosity, diffusion and electrochemical phenomena. McGraw-Hill, New York
Laidler KJ (1963) Reaction kinetics, homogeneous gas reactions, vol I. Pergamon Press, New York
Suhm MA (2009) Hydrogen bond dynamics in alcohol clusters. In: Rice SA (ed) Advances in chemical physics, vol 142. Wiley, Hoboken, pp 1–57
Böhmer R, Gainaru C, Richert R (2014) Structure and dynamics of monohydroxy alcohols—milestones towards their microscopic understanding, 100 years after Debye. Phys Rep 545:125–195
Engbersen JFJ, Engberts JBFN (1975) Water structure and its kinetic effects on the neutral hydrolysis of two acyl activated esters. J Am Chem Soc 97:1563–1568
Fábián A, Ruff F, Farkas Ö (2008) Mechanism of nucleophilic substitutions at phenacyl bromides with pyridines. A computational study of intermediate and transition state. J Phys Org Chem 21:988–996
Acknowledgements
This study was financially supported by the Russian Foundation for Basic Research (Project 17–03–00146–a).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
214_2017_2124_MOESM1_ESM.pdf
Cartesian coordinates for all calculated structures addressed in this study. Full table containing complexation energies and activation energies for all isomers of RC, TSA, I, TSB, SP (Table S1). Full table containing changes in enthalpy (∆H), entropy (T∆S), and Gibbs energies (∆G) relative to SR for compounds of the reactions 38 and 40 (kcal mole−1) calculated for 298 K (Table S2). (PDF 1720 kb)
Rights and permissions
About this article
Cite this article
Zabalov, M.V., Tiger, R.P. Specificities of application of the supermolecule method to the calculation of reaction mechanisms in a protonodonor medium. Ethylene carbonate aminolysis in methanol. Theor Chem Acc 136, 95 (2017). https://doi.org/10.1007/s00214-017-2124-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-017-2124-9