Abstract
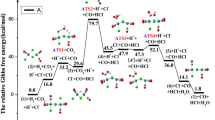
We systematically investigate the mechanisms of water-gas shift reaction (WGSR) on [Rh(EDTA)CO]− complex on the basis of density functional theory calculations. Two different reaction pathways have been considered: One is the synthesis of HCOOH, and the other is the direct formation of H2 from H2O and CO. The former offers new insights into the fundamental direct mechanism for WGSR. In this study, we combine with the energetic span model to study the catalytic activity of different active sites and two different reaction pathways. Our calculation results indicate that the formation of HCOOH mechanism is the energetically favorable pathway for the water-gas shift reaction on [Rh(EDTA)CO]− catalyst. Moreover, the Oc site acts as the most active site for the formation of HCOOH due to the highest value of TOF. NPA charges are calculated to shed further light on the properties leading up to the formation of HCOOH. Our work will be useful for developing the WGSR mechanism and designing better catalysts for WGSR.








Similar content being viewed by others
References
Navarro RM, Pena MA, Fierro JLG (2007) Chem Rev 107:3952–3991
Hao Y, Miharylov M, Ivanova E, Hadjiivanov K, Knozinger H, Gates BC (2009) J Catal 261:137–149
Spencer MS (1995) Catal Lett 32:9–13
Pulleri JK, Ting SW, Lam FLY, Hu C, Chan KY (2012) AIChE 2012 annual meeting, conference proceedings. American Institute of Chemical Engineers, New York, p 510e
Johnson TC, Morris DJ, Wills M (2010) Chem Soc Rev 39:81–88
Joo F (2008) ChemSusChem 1:805–808
Enthaler S (2008) ChemSusChem 1:801–804
Grabow LC, Mavrikakis M (2011) ACS Catal 1:365–384
Morooka S, Matubayasi N, Nakahara M (2008) J Phys Chem A 112:6950–6959
Rozenberg M, Loewenschuss A, Nielsen CJ (2015) J Phys Chem A 119:8497–8502
Avdeev V, Parmon VN (2011) J Phys Chem C 115:21755–21762
Chaoquan Hu, Jk Pulleri, Ting SW, Chan KY (2014) Int J Hydrogen Energy 39:381–390
Grasemann M, Laurenczy G (2012) Energy Environ Sci 5:8171–8181
Chaoquan Hu, Ting SW, Chan KY, Huang W (2012) Int J Hydrogen Energy 37:5956–5965
Mars P, Scholten JJF, Zwietering P (1963) Adv Catal 14:35–113
Trillo JM, Munuera G, Criado JM (1972) Catal Rev 7:51–86
Jessop PG, Ikariya T, Noyori R (1995) Chem Rev 95:259–272
Zhang JZ, Li Z, Wang H, Wang CY (1996) J Mol Catal A 112:9–14
Gassner F, Leitner W (1993) J Chem Soc Chem Commun 19:1465–1466
Bersworth CC (1954) Framingham, Mass
Suchecki SA, Mathews B, Augustyniak AW, Kumazawa H (2014) Ind Eng Chem Rec 53:14234–14240
Thomas SA, Gaillard JF (2015) J Phys Chem A 119:2878–2884
Garapati S, Burns CS, Rodriguez AA (2014) J Phys Chem B 118:12960–12964
Coskuner O, Jarvis EAA (2008) J Phys Chem A 112:2628–2633
Brien LC, Root HB, Wei CC, Jensen D, Shabestary N, Meo CD, Eder DJ (2015) Chem Educ 92:1547–1551
Dwyer FP, Garvan FL (1960) J Am Chem Soc 82:4823–4826
Herrmann WA (1990) Chem Ber 123:1963–1970
Gaussian 09, Revision A. 1, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA (2010) Gaussian Inc, Wallingford
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Andersson MP, Uvdal P (2005) J Phys Chem A 109:2937–2941
Häussermann U, Dolg M, Stoll H, Preuss H, Schwerdtfeger P, Pitzer RM (1993) Mol Phys 78:1211–1224
Kuchle W, Dolg M, Stoll H, Preuss H (1994) J Chem Phys 100:7535–7542
Leininger T, Nicklass A, Stoll H, Dolg M, Schwerdtfeger P (1996) J Chem Phys 105:1052–1059
Marenich AV, Cramer CJ, Truhlar DG (2009) J Phys Chem B 113:6378–6396
Fukui K (1981) Acc Chem Res 14:363–368
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Kozuch S, Shaik S (2010) Acc Chem Res 44:101–110
Kozuch S, Shaik SA (2006) J Am Chem Soc 128:3355–3365
Kozuch S, Shaik S (2008) J Phys Chem A 112:6032–6041
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20603021), the Natural Science Foundation of Shanxi (Grant No. 2013011009-6), the High School 131 Leading Talent Project of Shanxi, Undergraduate Training Programs for Innovation and Entrepreneurship of Shanxi Province (Grant No. 2013145 and Grant No. 2015537), Shanxi Normal University (SD2013CXCY-65, 105088), Teaching Reform Project of Shanxi Normal University (SD2013JGXM-51) and Graduate student innovation project (2016SY037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The researcher claims no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, Z., Guo, L., Liu, N. et al. Theoretical investigation of water-gas shift reaction catalyzed by water-soluble Rh(III)–EDTA complex. Theor Chem Acc 136, 53 (2017). https://doi.org/10.1007/s00214-017-2080-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-017-2080-4