Abstract
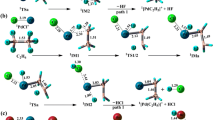
Density functional theory (DFT) calculations have been performed to investigate the reactivity of Th atom toward ethane C–H bond activation. To get the mechanism of the C–H bond activation by C2H6, a systematic DFT computational study is implemented. Comprehensive description of the reaction mechanism in the consideration of the possible spin states as well as analysis of the electronic factors offers detail information of C–H bond activation. The results indicate that the final reaction products of C–H breakage are the ThC2H3 and ThC2H2. The nature of the bonding evolution along the reaction pathways was explored using distinct analysis method including electron localization function, atoms in molecules and natural bond orbital. Reaction rate constants were computed between 298 and 1000 K at levels of variational transition state theory for the first time.








Similar content being viewed by others
References
Di Santo E, Santos M (2011) J Am Chem Soc 133:1955–1970
Santos M, Marc-alo J, Pires de Matos A, Gibson JK, Haire RG (2002) J Phys Chem A 106:7190–7194
Santos M, Marc-alo J, Leal JP, Pires de Matos A, Gibson JK, Haire RG (2003) Int J Mass Spectrom 228:457–465
Gibson JK, Haire RG, Santos M, Marc-alo J, Pires de Matos A (2005) J Phys Chem A 109:2768–2781
Gibson JK, Haire RG, Marc-alo J, Santos M, Pires de Matos A (2005) J Nucl Mater 344:24–29
Santos M, Pires de Matos A, Marc-alo J, Gibson JK, Haire RG, Tyagi R, Pitzer RM (2006) J Phys Chem A 110:5751–5759
Gibson JK, Haire RG, Santos M, Pires de Matos A, Marc-alo J (2008) J Phys Chem A 112:11373–11381
Marshall AG, Hendrickson CL, Jackson GS (1998) Mass Spectrom Rev 17:1–35
Li P, Niu WX, Gao T, Wang HY (2014) J Mol Model 20:2466
Niu WX, Zhang H, Li P, Gao T (2015) Int J Quantum Chem 115:6–18
Li P, Niu WX, Gao T, Wang HY (2014) ChemPhysChem 15:3078–3088
Li P, Niu WX, Gao T, Wang HY (2014) Int J Quantum Chem 114:760–768
Li P, Niu WX, Gao T (2015) J Radioanal Nucl Chem 304:489–499
Li P, Niu WX, Gao T (2014) RSC Adv 4:29806–29817
Li P, Niu WX, Tian XF, Gao T, Wang HY (2013) J Phys Chem A 117:3761–3770
DeAlmeida KJ, Duarte HA (2010) Organometallics 29:3735–3745
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas C, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 (Revision A.02). Gaussian Inc, Wallingford
Becke AD (1993) J Chem Phys 98:1372–1377
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1998) Matter Mater Phys 37:785–789
Adamo C, Barone V (1999) J Chem Phys 110:6158–6170
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6687
Perdew JP, Burke K, Wang Y (1996) Phys Rev B 54:16533–16539
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Phys Rev Lett 91:146401
Kuchle W, Dolg M, Stoll H, Preuss H (1996) J Chem Phys 100:7535–7542
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Blaudeau JP, McGrath MP, Curtiss LA, Radom L (1997) J Chem Phys 107:5016–5021
Lu T, Chen F (2012) J Comput Chem 33:580–592
Becke AD, Edgecombe KE (1990) J Chem Phys 92:5397–5403
Savin A, Nesper R, Wengert S, Fassler TR (1997) Angew Chem Int Ed Engl 36:1808–1832
Bader RFW (1990) Atoms in molecules. A quantum theory. Clarendon, Oxford
Reed AE, Weinhold F (1985) J Chem Phys 83:1736–1740
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Canneaux S, Bohr F, Henon E (2014) J Comput Chem 35:82
Carstensen HH, Dean AM (2009) J Phys Chem A 113:367
Ng M, Mok DKW, Lee EPF, Dyke JM (2013) J Comput Chem 34:545
Acknowledgements
We are very grateful for the Center of High Performance Computing at the Physics Discipline of Sichuan University providing computer time. We thank all those people for asking the critical questions which help to form this article.
Funding
Project was supported by the National Natural Science Foundation of China (Grant Nos. 21371160 and 21401173).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Q., Li, P., Gao, T. et al. Mechanistic aspects of the activation of C–H bond in C2H6 by Th atom: bonding analysis and reaction coefficients. Theor Chem Acc 135, 266 (2016). https://doi.org/10.1007/s00214-016-2015-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-2015-5