Abstract
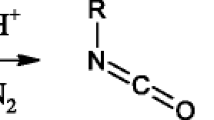
A new bimolecular pathway for a model aza-MBH reaction is presented and then explored in more details by DFT/M06-2X calculations. For this bimolecular pathway, explicit formic acid was considered in the rate-determining step showing the beneficial action from this additive, which plays a role as a co-catalyst. According to the current computations, this mechanistic cycle is a feasible pathway for the formation of the aza adduct and it explains the experimental detection of a key intermediate. A comparative analysis of the current results and previous ones reveals the substrate and medium dependence of the aza-MBH reaction. These factors lead to distinct pathways for the reaction, uncovering the complexity for conducting this reaction.







Similar content being viewed by others
References
Shi M, Wang F-J, Zhao M-X, Wei Y (2011) The chemistry of the Morita–Baylis–Hillman reaction. RSC Publishing, Cambrigde
Basavaiah D, Veeraraghavaiah G (2012) Chem Soc Rev 41:68–78
Wei Y, Shi M (2013) Chem Rev 113:6659–6690
Declerck V, Martinez J, Lamaty F (2009) Chem Rev 109:1–48
Masson G, Housseman C, Zhu J (2007) Angew Chem Int Ed 46:4614–4628
Hu F-L, Shi M (2014) Org Chem Front 1:587–595
Regiani T, Santos VG, Godoi MN, Vaz BG, Eberlin MN, Coelho F (2011) Chem Commun 47:6593–6595
Verma P, Verma P, Sunoj RB (2014) Org Biomol Chem 12:2176–2179
Mansilla J, Saá JM (2010) Molecules 15: 709–734
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Cantillo D, Kappe CO (2010) J Org Chem 75:8615–8626
Zhao Y, Truhlar DG (2008) Acc Chem Res 41:157–167
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, MDeFrees DJ, Pople JA (1982) J Chem Phys 77:3654–3665
Hratchian HP, Schlegel HB (2005) J Chem Theory Comput 1:61–69
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Gonzalez C, Schlegel HB (1991) J Chem Phys 95:5853–5860
Marenich AV, Cramer CJ, Truhlar DG (2009) J Phys Chem B 113:6378–6396
Bode B, Gordon MS (1998) J Mol Graph Model 16:133–138
Amarante GW, Milagre HMS, Vaz BG, Ferreira BRV, Eberlin MN, Coelho F (2009) J Org Chem 74:3031–3037
Zhao GJ, Han KL (2012) Acc Chem Res 45:404–413
Roy D, Patel C, Sunoj RB (2009) J Org Chem 74:6936–6943
Jones CE, Turega SM, Clarke ML, Philp D (2008) Tetrahedron Lett 49:4666–4669
Robiette R, Aggarwal VK, Harvey JN (2007) J Am Chem Soc 129:15513–15525
Price KE, Broadwater SJ, Jung HM, McQuade DT (2005) Org Lett 7:147–150
Carrasco-Sanchez V, Simirgiotis MJ, Santos LS (2009) Molecules 14:3989–4021
Acknowledgments
A.P.d.L.B. is thankful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Grant #2013/22235-0 and the support of the Computation Center of the University of São Paulo (LCCA-USP). FC thanks FAPESP for research Grants #2013/10449-5 and 2013/07600-3 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for research fellowship. A.A.C.B. thanks FAPESP for research Grant #2015/01491-3.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Lima Batista, A.P., Coelho, F. & Braga, A.A.C. DFT exploration of mechanistic pathways of an aza-Morita–Baylis–Hillman reaction. Theor Chem Acc 135, 186 (2016). https://doi.org/10.1007/s00214-016-1946-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1946-1