Abstract
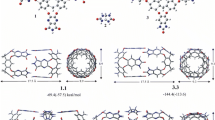
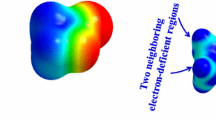
A theoretical study has been carried out on the encapsulation of heterodimers and homodimers of p-methylbenzoic acid, p-ethylbenzoic acid, p-methylbenzamide, and p-ethylbenzamide molecules in reversible capsules with a very limited cavity. The drastic compression of the guests in the capsules has been studied by density functionally theory employing the M06-2X and ωB97X-D functionals with the 6-31G(d,p) basis set following preliminary calculations by the fast ONIOM[M06-2X/6-31G(d,p):PM6] methodology. Both functionals are in agreement with respect to the geometry, the interaction energies between the monomers and the relative ordering of the isomers. We found that encapsulation is favorable even for the larger p-ethyl compounds, but complexes of encapsulated dimers lie more than 4 kcal/mol above complexes with two non-interacting encapsulated monomers. The monomers prefer to be by themselves in the host. This is the reason why the present encapsulated dimers have not been found experimentally. The relative stability of the encapsulated complexes is reversed compared to complexes in a large cavity (Tzeli et al. in J Am Chem Soc 133:16977, 2012). This shows the possibility of separation of competitive guests via reversible encapsulation under appropriate conditions.




Similar content being viewed by others
References
(1996) Comprehensive supramolecular chemistry. In: Atwood JL, Davies JED, MacNicol DD, Vögtle F, Lehn JM (eds) Supramolecular reactivity and transport: bioinorganic systems, vol 5. Pergamon, Oxford
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
(2000) Recent theoretical and experimental advances in hydrogen bonded clusters. In: Xantheas SS. Kluwer Academic Publishers, NATO ASI Series C: Mathematical and Physical Sciences, vol 561
Desiraju GR, Steiner T (2001) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Cram DJ, Cram JM (1994) Container molecules and their guests, monographs in supramolecular chemistry. In: Stoddart JF (Ed) Royal Society of Chemistry, vol 4, Cambridge
Sherman JC (1995) Tetrahedron 51:3395–3422
Rebek J, Jr (1996) Chem Soc Rev 96:255–264
Warmuth R (2000) J Incl Phenom Mol Recognit Chem 37:1–38
Fochi F, Jacopozzi P, Wegelius E, Rissanen K, Cozzini P, Marastoni E, Fisicaro E, Manini P, Fokkens R, Dalcanale E (2001) J Am Chem Soc 123:7539–7552
Kang J, Hilmersson G, Santamaría J, Rebek J Jr (1998) J Am Chem Soc 120:3650–3656
van Wageningen AMA, Timmerman P, van Duynhoven JPM, Verboom W, van Veggel FCJM, Reinhoudt DN (1997) Chem Eur J 3:639–654
Conn MM, Rebek J Jr (1997) Chem Rev 97:1647–1668
Jasat A, Sherman JC (1999) Chem Rev 99:931–967
MacGillivray LR, Atwood JL (1999) Angew Chem Int Ed 38:1018–1033
Hof F, Craig SL, Nuckolls C, Rebek J Jr (2002) Angew Chem Int Ed 41:1488–1508
Imaoka T, Kawana Y, Kurokawa T, Yamamoto K (2013) Nat Commun 4:2581
Ajami D, Dube H, Rebek J Jr (2011) J Am Chem Soc 133:9689–9691
Ajami D, Tolstoy PM, Dube H, Odermatt S, Koeppe B, Guo J, Limback HH, Rebek J Jr (2011) Angew Chem Int Ed 50:528–531
Jiang W, Tiefenbacher K, Ajami D, Rebek J Jr (2012) Chem Sci 3:3022–3025
Tzeli D, Theodorakopoulos G, Petsalakis ID, Ajami D, Rebek J Jr (2011) J Am Chem Soc 133:16977–16985
Tzeli D, Petsalakis ID, Theodorakopoulos G, Ajami D, Rebek J Jr (2013) Int J Quant Chem 113:734–739
Tzeli D, Petsalakis ID, Theodorakopoulos G, Ajami D, Jiang W, Rebek J Jr (2012) Chem Phys Lett 548:55–59
Tzeli D, Petsalakis ID, Theodorakopoulos G (2013) Chem Phys Lett 573:48–55
Heinz T, Rudkevich DM, Rebek J Jr (1998) Nature 394:764–766
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Zhao Y, Truhlar DG (2008) Acc Chem Res 41:157–167
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615
Curtiss LA, McGrath MP, Blaudeau JP, Davis NE, Binning RC Jr, Radom L (1995) J Chem Phys 103:6104–6113
Tzeli D, Petsalakis ID, Theodorakopoulos G (2011) Phys Chem Chem Phys 13:11965–11975
Dapprich S, Komáromi I, Byun KS, Morokuma K, Frisch MJ (1999) J Mol Struct (Theochem) 462:1–21
Vreven T, Morokuma K, Farkas Ö, Schlegel HB, Frisch MJ (2003) J Comp Chem 24:760–769
Vreven T, Morokuma K (2006) Ann Rep Comp Chem 2:35–50
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Xantheas SS (1994) J Chem Phys 104:8821–8824
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Wallingford CT, Gaussian 09, Revision A1
Mitra T, Jelfs KE, Schmidtmann M, Ahmed A, Chong SY, Adams DJ, Cooper AI (2013) Nat Chem 5:276–281
Acknowledgments
Financial support from GSRT and the EC through the European Fund for Regional Development, NSRF 2007–2013 action Development of Research Centers—KPHPIS, project “NewMultifunctional Nanostructured Materials and Devices—POLYNANO” to GT and IDP is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tzeli, D., Petsalakis, I.D., Theodorakopoulos, G. et al. The role of the host–guest interactions in the relative stability of compressed encapsulated homodimers and heterodimers of amides and carboxylic acids. Theor Chem Acc 133, 1503 (2014). https://doi.org/10.1007/s00214-014-1503-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-014-1503-8