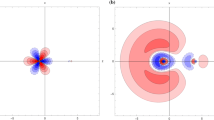
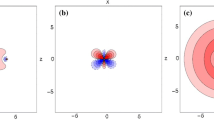
Abstract
The electronic structure of the EuF molecule is investigated using a four-component relativistic general open-shell configuration interaction method. All low-lying excited states below 3.0 eV are characterized by applying the f-shell Omega decomposition method, which was proposed by the present authors to analyze the electronic spectra of GdF. The ground states are ninefold degenerate and are expressed in the present terminology as X 4[(4f 7)(6s 1)]Ω. The superscript (4) here denotes the maximum Ω value. The electronic angular momentum projected onto the molecular axis (Ω) runs from 4 to −4, and the electronic configuration is represented symbolically by the gross atomic orbital populations of the Eu moiety (4f)7(6s)1. These features are consistent with the term X 9Σ that is assigned experimentally in the LS-coupling scheme. Similarly, the sevenfold degenerate first excited states are characterized as a 3[(4f 7)(6s 1)]Ω, corresponding to the experimentally assigned a 7Σ term. Dmitriev et al. observed three excited states Ω2, Ω1, and Ω3 above a 7Σ term. The three calculated excited states, A 4[(4f 7)1/2(6p 1)3/2 + …]2, A 4[(4f 7)−1/2(6p 1)3/2 + …]1, and B 4[(4f 7)5/2(6p 1+5d 1)1/2 + …]3, are, respectively, the most plausible identifications of the Ω2, Ω1, and Ω3 given by Dmitriev et al. These three states have large oscillator strengths with the X and a families.

Similar content being viewed by others
References
Cotton S (2006) Lanthanide and actinide chemistry. John Wiley and Sons, Chichester
Bünzli J-CG (2010) Chem Rev 110:2729–2755
Binnemans K (2009) Chem Rev 109:4283–4374
Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD (2010) Chem Rev 110:2960–3018
Field RW (1982) Ber Bunsenges Phys 86:771–779
Schall H, Dulick M, Field RW (1987) J Chem Phys 87:2898–2912
Kaledin LA, Linton C, Clarke TE, Field RW (1992) J Mol Spectrosc 154:417–426
Kaledin LA, McCord JE, Heaven MC (1992) J Mol Spectrosc 170:166–171
Schamps J, Benchheikh M, Barthelat J-C, Field RW (1993) J Chem Phys 103:8004–8013
Kaledin LA, Bloch JC, McCarthy MC, Shenyavskaya EA, Field RW (1996) J Mol Spectrosc 176:148–161
Kaledin AL, Heaven MC, Field RW, Kaledin LA (1996) J Mol Spectrosc 179:310–319
Ren J, Whangbo M-H, Dai D, Li L (1998) J Chem Phys 108:8479–8485
Dolg M, Stoll H (1989) Theor Chim Acta 75:369–387
Titov AV, Mosyagin NS, Ezhov VF (1996) Phys Rev Lett 77:5346–5349
Lesar A, Muri G, Hodošček M (1998) J Phys Chem A 102:1170–1176
Cao X, Liu W, Dolg M (2002) Sci China, Ser B 45:91–96
Fahs H, Allouche AR, Korek M (2002) J Chem Phys 117:3715–3720
Wang SG, Schwarz WHE (1995) J Phys Chem 99:11687–11695
Heiberg H, Gropen O, Laerdahl JK, Swang O, Wahlgren U (2003) Theor Chem Acc 110:118–125
Hülsen M, Dolg M, Link P, Ruschewitz U (2011) Theor Chem Acc 129:367–379
Quiney HM, Skaane H, Grant IP (1998) J Phys B 31:L85–L96
Laerdahl JK, Fægri K Jr, Visscher L, Saue T (1998) J Chem Phys 109:10806–10817
Wasada-Tsutsui Y, Watanabe Y, Tatewaki H (2009) Int J Quantum Chem 109:1874–1885
Liu W, Dolg M, Li L (1998) J Chem Phys 108:2886–2895
Dolg M, Liu W, Kalvoda S (2000) Int J Quantum Chem 76:359–370
Moriyama H, Watanabe Y, Nakano H, Tatewaki H (2008) J Phys Chem A 112:2683–2692
Moriyama H, Tatewaki H, Watanabe Y, Nakano H (2009) Int J Quantum Chem 109:1898–1904
Moriyama H, Watanabe Y, Nakano H, Yamamoto S, Tatewaki H (2010) J Chem Phys 132:124310 (9 pp)
Wasada-Tsutsui Y, Watanabe Y, Tatewaki H (2007) J Phys Chem A111:8877–8883
Tatewaki H, Yamamoto S, Watanabe Y, Nakano H (2008) J Chem Phys 128:214901 (8 pp)
Tatewaki H, Matsuoka O (1997) J Chem Phys 106:4558–4565
Tatewaki H, Watanabe Y, Yamamoto S, Miyoshi E (2006) J Chem Phys 125:044309 (9 pp)
Yamamoto S, Tatewaki H, Saue T (2008) J Chem Phys 129:244505 (8 pp)
Yamamoto S, Tatewaki H (2011) J Chem Phys 134:164310 (11 pp)
Yamamoto S, Tatewaki H (2012) Comput Theoret Chem 980:37–43
Visser O, Visscher L, Aerts PJC, Nieuwpoort WC (1992) J Chem Phys 96:2910–2919
Zmbov KF, Margrave JL (1967) J Inorg Nucl Chem 29:59–63
Kleinschmidt PD, Lau KH, Hildenbrand DL (1981) J Chem Phys 74:653–660
Dmitriev YN, Kaledin LA, Kobylyansky AI, Kulikov AN, Shenyavskaya EA, Gurvich LV (1987) Acta Physica Hungarica 61:51–54
Gurvich LV, Dmitriev YuN, Kaledin LA, Kobylyanskii AI, Kulikov AN, Shenyavskaya EA (1989) Bull Acad Sci USSR (Phys Ser) 53:75–79
Kaledin LA, Heaven MC, Field RW (1999) J Mol Spectrosc 193:285–292
Koga T, Tatewaki H, Matsuoka O (2001) J Chem Phys 115:3561–3565. See also http://www.nsc.nagoya-cu.ac.jp/~htatewak/english.html
Koga T, Tatewaki H, Matsuoka O (2002) J Chem Phys 117:7813–7814. See also http://www.nsc.nagoya-cu.ac.jp/~htatewak/english.html
Andzelm J, Kłobukowski M, Radio-Andzelm E, Sakai Y, Tatewaki H. (1984) In: Huzinaga S (ed) Gaussian basis sets for molecular calculations. Elsevier, Amsterdam
Lee YS, McLean AD (1982) J Chem Phys 76:735–736
Ishikawa Y, Binning RC Jr, Sando KM (1983) Chem Phys Lett 101:111–114
Stanton RE, Havriliak S (1984) J Chem Phys 81:1910–1918
Visscher L, Dyall KG (1997) At Data Nucl Data Tables 67:207–224
Jensen HJAa, Saue T, Visscher L et al (2004) DIRAC, a relativistic ab initio electronic structure program, Release DIRAC 04.0; http://dirac.chem.sdu.dk
Huzinaga S, Arnau C (1971) J Chem Phys 54:1948–1951
Bauschlicher CW Jr (1980) J Chem Phys 72:880–885
Mulliken RS (1955) J Chem Phys 23:1833–1840
Rösch N (1983) Chem Phys 80:1–5
Martin WC, Zalubas R, Hagan L (1978) In Natl Stand Ref Data Ser, US Natl Bur Stand 60:199–203
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, S., Tatewaki, H. & Moriyama, H. Electronic spectra of EuF studied by a four-component relativistic configuration interaction method. Theor Chem Acc 131, 1230 (2012). https://doi.org/10.1007/s00214-012-1230-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1230-y