Abstract
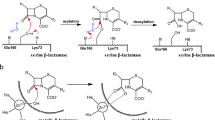
The Schiff base formation catalyzed by type I dehydroquinate dehydratase (DHQD) from Salmonella enterica has been studied by molecular docking, molecular dynamics simulation, and quantum chemical calculations. The substrate locates stably a similar position as the Schiff base intermediate observed in the crystal structure and forms strong hydrogen bonds with several active site residues. This binding mode is different from that of several other Schiff base enzymes. Then, the quantum chemical model has been constructed and the fundamental reaction pathways have been explored by performing quantum chemical calculation. The energy barrier of the previously proposed reaction pathway is calculated to be 30.7 kcal/mol, which is much higher than the experimental value of 14.3 kcal/mol of the whole dehydration reaction by type I DHQD from S. enterica. It means that this pathway is not favorable in energy. Therefore, a new and unexpected reaction pathway has been investigated with the favorable and reasonable energy barrier of 12.1 kcal/mol. The complicated role of catalytic His143 residue has also been elucidated that it mediates two proton transfers to facilitate the reaction. Moreover, the similarity and the difference between these two reaction pathways have been analyzed in detail. The new structural and mechanistic insights may direct the design of the inhibitors of type I dehydroquinate dehydratase as non-toxic antimicrobials, antifungals, and herbicides.






Similar content being viewed by others
References
Bentley R (1990) Crit Rev Biochem Mol Biol 25:307–384
Marques MR, Pereira JH, Oliveira JS, Basso LA, de Azevedo WF, Santos DS, Palma MS (2007) Curr Drug Targets 8(3):445–457
Noble M, Sinha Y, Kolupaev A, Demin O, Earnshaw D, Tobin F, West J, Martin JD, Qiu CY, Liu WS, DeWolf WE, Tew D, Goryanin II (2006) Biotechnol Bioeng 95(4):560–573. doi:10.1002/Bit.20772
Butler JR, Alworth WL, Nugent MJ (1974) J Am Chem Soc 96(5):1617–1618
Gourley DG, Shrive AK, Polikarpov I, Krell T, Coggins JR, Hawkins AR, Isaacs NW, Sawyer L (1999) Nat Struct Biol 6(6):521–525
Kleanthous C, Deka R, Davis K, Kelly SM, Cooper A, Harding SE, Price NC, Hawkins AR, Coggins JR (1992) Biochem J 282:687–695
White PJ, Young J, Hunter IS, Nimmo HG, Coggins JR (1990) Biochem J 265(3):735–738
Harris J, Kleanthous C, Coggins JR, Hawkins AR, Abell C (1993) Chem Commun 13:1080–1081
Leech AP, James R, Coggins JR, Kleanthous C (1995) J Biol Chem 270(43):25827–25836
Light SH, Minasov G, Shuvalova L, Duban ME, Caffrey M, Anderson WF, Lavie A (2011) J Biol Chem 286(5):3531–3539
Burgi HB, Dunitz JD, Lehn JM, Wipff G (1974) Tetrahedron 30(12):1563–1572
Blom N, Sygusch J (1997) Nat Struct Biol 4(1):36–39
Soares da Costa TP, Muscroft-Taylor AC, Dobson RCJ, Devenish SRA, Jameson GB, Gerrard JA (2010) Biochimie 92(7):837–845
Pohl E, Pauluhn A, Ahmed H, Lorentzen E, Buchinger S, Schomburg D, Siebers B (2008) Proteins 72(1):35–43
Chaudhuri S, Lambert JM, Mccoll LA, Coggins JR (1986) Biochem J 239(3):699–704
Deka RK, Kleanthous C, Coggins JR (1992) J Biol Chem 267(31):22237–22242
Leech AP, Boetzel R, McDonald C, Shrive AK, Moore GR, Coggins JR, Sawyer L, Kleanthous C (1998) J Biol Chem 273(16):9602–9607
Li H, Robertson AD, Jensen JH (2005) Proteins 61(4):704–721
Bas DC, Rogers DM, Jensen JH (2008) Proteins 73(3):765–783
Olsson MHM, Søndergaard CR, Rostkowski M, Jensen JH (2011) J Chem Theory Comput 7(2):525–537
Søndergaard CR, Olsson MHM, Rostkowski M, Jensen JH (2011) J Chem Theory Comput 7(7):2284–2295
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) J Comput Chem 30(16):2785–2791
Case DA, Cheatham TE, Darden T, Gohlke H, Luo R, Merz KM, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) J Comput Chem 26(16):1668–1688
Case Dad TA, Cheatham TE, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Pearlman DA, Crowley M, Walker RC, Zhang W, Wang B, Hayik S, Roitberg A, Seabra G, Wong KF, Paesani F, Wu X, Brozell S, Tsui V, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Beroza P, Mathews DH, Schafmeister C, Ross WS, Kollman PA (2006) Amber 9. University of California, San Francisco
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79(2):926–935
Miyamoto S, Kollman PA (1992) J Comput Chem 13(8):952–962
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) J Comput Phys 23(3):327–341
Frisch MJT, G. W, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JJA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision E01. Gaussian, Inc., Wallingford CT
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255(4–6):327–335
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55(1):117–129
Miertus S, Tomasi J (1982) Chem Phys 65(2):239–245
Noodleman L, Lovell T, Han WG, Li J, Himo F (2004) Chem Rev 104(2):459–508
Cui FC, Pan XL, Liu W, Liu JY (2011) J Comput Chem 32:3068–3074
Himo F, Eriksson LA, Maseras F, Seigbahn PEM (2000) J Am Chem Soc 122:8031–8036
Cui FC, Pan XL, Liu JY (2010) J Phys Chem B 114(29):9622–9628
Himo F, Chen SL, Marino T, Fang WH, Russo N (2008) J Phys Chem B 112(8):2494–2500
Himo F, Liao RZ, Yu JG (2010) Proc Natl Acad Sci USA 107(52):22523–22527
Himo F, Liao RZ, Yu JG (2010) J Phys Chem B 114(7):2533–2540
Liao RZ, Yu JG, Himo F (2011) J Chem Theory Comput 7(5):1494–1501
Highbarger LA, Gerlt JA, Kenyon GL (1996) Biochemistry 35(1):41–46
Reed AE, Weinhold F (1985) J Chem Phys 83(4):1736–1740
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83(2):735–746
Acknowledgments
This work was supported by the Major State Basic Research Development Programs of China (2011CBA00701), the National Natural Science Foundation of China (20973049), and Development Program for Outstanding Young Teachers in Harbin Institute of Technology (HITQNJS.2009.069).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pan, Q., Yao, Y. & Li, ZS. New insights into the mechanism of the Schiff base formation catalyzed by type I dehydroquinate dehydratase from S. enterica . Theor Chem Acc 131, 1204 (2012). https://doi.org/10.1007/s00214-012-1204-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1204-0