Abstract
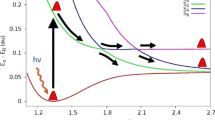
For studying the adiabatic and nonadiabatic mechanisms of the ClO (X 2Π) + ClO (X 2Π) → ClOOCl → 2Cl (2 P u) + O2 (X 3Σ − g ) reaction (1) and the ClO (X 2Π) + ClO (X 2Π) → ClOOCl → Cl2 (X 1Σ + g ) + O2 (X 3Σ − g ) reaction (2), we calculated, by partial geometry optimizations under the C2 constraint, the O–O and O–Cl dissociation potential energy curves (PECs) from the five low-lying states of ClOOCl at the CASPT2 level. The CASSCF minimum-energy crossing point (MECP) between the potential energy surfaces of the 1 1A ground state [correlating with the product of reaction (1)] and the 1 3B state [correlating with the product of reaction (2)] states was also determined. Based on the CAS calculation results (PECs, energies, and spin–orbit coupling at the MECP), we predict that reaction (1) occurs along pathway 1: ClO (X 2Π) + ClO (X 2Π) → ClOOCl (1 1A) → 2Cl (2 P u) + O2 (X 3Σ − g ) and that reaction (2) occurs along pathway 2: ClO (X 2Π) + ClO (X 2Π) → ClOOCl (1 1A) → 1 1A/1 3B MECP (142.4 cm−1) → ClOOCl (1 3B) → Cl2 (X 1Σ + g ) + O2 (X 3Σ − g ). The needed energies (relative to the reactant) for pathways 1 and 2 are predicted to be 5.3 and 11.1 kcal/mol, respectively, which indicates that reaction (1) is more favorable than reaction (2). The present work supports the traditional photochemical model for ozone degradation: ClOOCl (1 1A), formed by two ClO (X 2Π), can directly produce O2 plus two Cl atoms.


Similar content being viewed by others
References
Molina LT, Molina MJ (1987) J Phys Chem 91:433–436
Cox RA, Hayman GD (1988) Nature 332:796–800
Sander SP, Friedl RR, Yung YL (1989) Science 245:1095–1098
World Meteorological Organization (WMO) (2006) Scientific assessment of ozone depletion. WMO, global ozone research and monitoring project, report no. 50: Geneva, Switzerland, 2007, http://ozone.unep.org/Assessment_Panels/SAP/Scientific_Assessment_2006. Accessed Feb 2007
von Hobe M, Salawitch RJ, Canty T, Keller-Rudek H, Moortgat GK, Grooss J-U, Muller R, Stroh F (2007) Atmos Chem Phys 7:3055–3069
Santee ML, Sander SP, Livesey NJ, Froidevaux L (2010) PNAS 107(15):6588–6593. doi:10.1073/pnas.0912659107
Ferracci V, Rowley DM (2010) Phys Chem Chem Phys 12:11596–11608
Sander SP, Ravishankara AR, Golden DM, Kolb CE, Kurylo MJ, Molina MJ, Moortgat GK, Finlayson-Pitts BJ, Wine PH, Huie RE, Orkin VL (2006) Chemical kinetics and photochemical data for use in atmospheric studies. Evaluation no. 15, JPL publication 06-2. Jet Propulsion Laboratory, Pasadena
Trolier M, Mauldin RL III, Ravishankara AR (1990) J Phys Chem 94:4896–4907
Birk M, Friedl RR, Cohen EA, Pickett HM, Sander SP (1989) J Chem Phys 91:6588–6597
Jacobs J, Kronberg M, Müller HSP, Willner H (1994) J Am Chem Soc 116:1106–1114
Moore TA, Okumura M, Seale JW, Minton TK (1999) J Phys Chem A 103:1691–1695
Pope FD, Hansen JC, Bayes KD, Friedl RR, Sander SP (2007) J Phys Chem A 111:4322–4332
von Hobe M, Stroh F, Beckers H, Benter T, Willner H (2009) Phys Chem Chem Phys 11:1571–1580
Wilmouth DM, Hanisco TF, Stimpfle RM, Anderson JG (2009) J Phys Chem A 113:14099–14108
Papanastasiou DK, Papadimitriou VC, Fashey DW, Burkholder JB (2009) J Phys Chem A 113:13711–13726
Chen H-Y, Lien C-Y, Lin W-Y, Lee YT, Lin JJ (2009) Science 324:781–784
Lien C-Y, Lin W-Y, Chen H-Y, Huang W-T, Jin B, Chen C, Lin JJ (2009) J Chem Phys 131:174301
Jin B, Chen I-C, Huang W-T, Lien C-Y, Guchhait N, Lin JJ (2010) J Phys Chem A 114:4791–4797
Huang W-T, Chen AF, Chen I-C, Tsai C-H, Lin JJ (2011) Phys Chem Chem Phys 13:8195–8203
McGrath MP, Clemitshaw KC, Rowland FS, Hehre WJ (1990) J Phys Chem 94:6126–6132
Rendell AP, Lee TJ (1991) J Chem Phys 94:6219–6228
Lee TJ, Rohlfing CM, Rice JE (1992) J Chem Phys 97:6593–6605
Stanton JF, Bartlett RJ (1993) J Chem Phys 98:9335–9339
Kaledin AL, Morokuma K (2000) J Chem Phys 113:5750–5762
Toniolo A, Persico M, Pitea D (2000) J Phys Chem A 104:7278–7283
Toniolo A, Granucci G, Inglese S, Persico M (2001) Phys Chem Chem Phys 3:4266–4279
Tomasello P, Ehara M, Nakatsuji H (2002) J Chem Phys 116:2425–2432
Tomasello P, Ehara M, Nakatsuji H (2003) J Chem Phys 118:5811–5820
Peterson KA, Francisco JS (2004) J Chem Phys 121:2611–2616
Ončák M, Šištík L, Slavíček P (2010) J Chem Phys 133:174303
Grein F (2011) Chem Phys 382:34–40
Zhu RS, Lin MC (2003) J Chem Phys 118:4094–4106
Andersson K, Roos BO (1995) Multiconfigurational second-order perturbation theory. In: Yarkony DR (ed) Modern electronic structure theory, part 1. World Scientific, Singapore, p 55
Andersson K, Roos BO (1993) Int J Quantum Chem 45:591–607
Roos BO (1987) The complete active space self-consistent field method and its applications in electronic structure calculations. In: Lawley KP (ed) Advances in chemical physics. Wiley, New York, pp 399–445
Karlström G, Lindh R, Malmqvist P-Å, Roos BO, Ryde U, Veryazov V, Widmark P-O, Cossi M, Schimmelpfennig B, Neogrady P, Seijo L (2003) Comput Mater Sci 28:222
Aquilante F, De Vico L, Ferré N, Ghigo G, Malmqvist P-Å, Neogrády P, Pedersen TB, Pitoňák M, Reiher M, Roos BO, Serrano-Andrés L, Urban M, Veryazov V, Lindh R (2010) J Comput Chem 31:224–247
Almlof J, Taylor PR (1987) J Chem Phys 86:4070–4077
Pierloot K, Dumez B, Widmark P-O, Roos BO (1995) Theoret Chem Acc 90:87–114
Widmark P-O, Malmqvist P-Å, Roos BO (1990) Theoret Chem Acc 77:291–306
Widmark P-O, Persson BJ, Roos BO (1991) Theoret Chem Acc 79:419–432
Li J, Hao Y, Yang J, Zhou C, Mo Y (2007) J Chem Phys 127:104307
Acknowledgments
We appreciate the financial support for this work that was provided by National Natural Science Foundation of China through Contract No. 20773161. We thank the anonymous referees for their constructive suggestions to improve the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, Q., Dong, H. & Huang, MB. Multiconfigurational study on the synchronous mechanisms of the ClO self-reaction leading to Cl or Cl2 . Theor Chem Acc 131, 1194 (2012). https://doi.org/10.1007/s00214-012-1194-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1194-y