Abstract
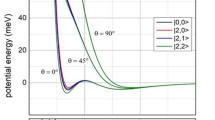
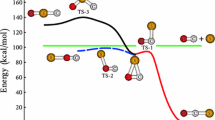
To understand the title reaction, the MRCI, CCSD, CCSD(T), and DFT calculations have been done. A large domain of the ground-state potential energy surface has been explored including the activation energy barrier to form the triatomic complex, two stable intermediate complexes, V[NO] and NVO, the transition state connecting these two conformers, and the detachment of the nitrogen atom. We compared this reaction with the similar ones involving the Sc and Ti atoms. The activation barrier to form the VNO complex made from the ionic-covalent coupling decreases to approach the experimental data when the electron correlation effect is better included as in the Sc and Ti systems. The transition state connecting the two conformers was calculated to be higher than in the Sc and Ti cases probably due to larger number of nonbonding valence electrons and is probably too high with respect to the reactant energy level to allow the interconversion between the two conformers in the VNO. The direct concerted substitution (abstraction) reaction is improbable because this process will have to overcome a too high potential barrier. We have also found the transition state connecting two conformers of ScNO.




Similar content being viewed by others
References
Ledentu V, Rahmouni A, Jeung GH, Lee YS (2004) Bull Korean Chem Soc 25:1645–1647
Kim KH, Lee YS, Kim D, Kim KS, Jeung GH (2002) J Phys Chem A 106:9600–9605
Kim KH, Lee YS, Moon JH, Kim Y, Jeung GH (2002) J Chem Phys 117:8385–8390
Naulin C, Costes M (1999) Chem Phys Lett 310:231–239
Luc P, Vetter R (2001) J Chem Phys 115:11106–11117
Jeung GH, Luc P, Vetter R, Kim KH, Lee YS (2002) Phys Chem Chem Phys 4:596–600
Naulin C, Hedgecock IM, Costes M (1997) Chem Phys Lett 266:335–341
Vetter R, Naulin C, Costes M (2000) Phys Chem Chem Phys 2:643–649
Ishida M, Yamashiro R, Matsumoto Y, Honma K (2006) J Chem Phys 124:204316-1–7
Karlstrőm G, Lindh R, Malmqvist PǺ, Roos BO, Ryde U, Veryazov V, Widmark PO, Cossi M, Schimmelpfennig B, Neogrady P, Seijo L (2003) Comput Mater Sci 28:222–239
Werner HJ, Knowles PJ, Lindh R, Manby FR, Schűtz M, and others (2006) MOLPRO, version 2006.1, a package of ab initio programs, see http://www.molpro.net
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian, Inc., Wallingford
Becke AD (1993) J Chem Phys 98:5648–5652
NIST Chemistry WebBook (2011) National Institute of Standards and Technology (NIST), Gaithersburg, MD. http://webbook.nist.gov/chemistry/. Accessed 8 Mar 2011
Pedley JB, Marshall EM (1983) J Phys Chem Ref Data 12:967–1031
Siegel MW, Celotta RJ, Hall JL, Levine J, Bennett RA (1972) Phys Rev A 6:607–631
Lee D, Lee YS, Hagebaum-Reignier D, Jeung GH (2006) Chem Phys 327:406–414
Jeung GH (2006) Theor Chem Acc 116:450–455
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure IV. Constants of diatomic molecules. Van Nostrand Rheinhold, New York
Acknowledgments
This work was supported by grants (2009-0084918, 2010-0001632) from National Research Foundation, a national grant from KETEP of MKE, the EEWS program of KAIST, and CNRS. Computational resources were provided by the supercomputing center of the KISTI (KSC-2011-C2-18).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Shigeru Nagase on the occasion of his 65th birthday and published as part of the Nagase Festschrift Issue.
Rights and permissions
About this article
Cite this article
Lee, Dk., Park, Y.C., Lee, Y.S. et al. Theoretical study of the V(4F) + NO(2Πr) → VO(4Σ−) + N(4S°) reaction compared with the Sc(2D) and Ti(3F) cases. Theor Chem Acc 130, 563–570 (2011). https://doi.org/10.1007/s00214-011-1061-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-011-1061-2