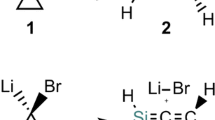
Abstract
The mechanisms of [2 + 2 + 2] reactions of three ethynes and monosilaethylenes to form benzene and 1,3,5-trisilacyclohexane were studied by ab initio MO methods. The reaction mechanisms were analyzed by configuration interaction/localized molecular orbital/CASSCF calculations. Although the [2 + 2 + 2] reaction of ethyne is typically “homologous” concerted, that of monosilaethylene is polarized (ionic-cyclic) one-step reaction. In addition, the aromaticity along the intrinsic reaction coordinate pathway was studied using the index of deviation from aromaticity. Although the transition state of trimerization of ethyne does not have an aromatic nature for the σ- and π-bonds formation system, the crossing point of the σ-bond formation and π-bond breaking shows an aromatic nature.







Similar content being viewed by others
References
Woodward RB, Hoffmann R (1970) The conservation of orbital symmetry. Verlage Chemie, Weinheim
Berthelot M (1866) C R Acad Sci 62:905. doi:10.1002/jlac.18661390303 (see: http://www.biodiversitylibrary.org/item/23761#page/913/mode/1up)
Berthelot M (1866) Ueber die Einwirkung der Hitze auf einige Kohlenwasserstoffe. Justus Liebigs Annalen der Chemie 139:272–282. (see: http://www.archive.org/details/annalenderchemi11liebgoog)
Zimmerman HE (1971) Acc Chem Res 4:272
Houk KN, Gandour R, Strozier R, Rondan N, Paquette L (1979) J Am Chem Soc 101:6797
Bach RD, Wolber GJ, Schleyer HB (1985) J Am Chem Soc 107:2837
Ioffe A, Shaik S (1992) J Chem Soc Perkin Trans 2:2101
Wagenseller PE, Birney DM, Roy D (1995) J Org Chem 60:2853
Jiao H, Schleyer PvR (1998) J Phys Org Chem 11:655
Morao I, Cossio F (1999) J Org Chem 64:1868
Sawicka D, Wilsey S, Houk KN (1999) J Am Chem Soc 121:864
Sawicka D, Li Y, Houk KN (1999) J Chem Soc Perkin Trans 2:2349
Cioslowski J, Liu G, Moncrieff D (2000) Chem Phys Lett 316:536
Havenith R, Fowler P, Jenneskens L, Steiner E (2003) J Phys Chem A 107:1867
Santos JC, Polo V, Andres J (2005) Chem Phys Lett 406:393
Eichberg MJ, Houk KN, Lehmann J, Leonard PW, Marker A, Norton JE, Sawicka D, Vollhardt KPC, Whitener GD, Wolff S (2007) Angew Chem Int Ed 46:6894
Donoso-Tauda O, Aizman A, Escobar CA, Santos JC (2009) Chem Phys Lett 469:219
Sakai S (2002) J Phys Chem A 106:10370
Sakai S (2002) J Phys Chem A 106:11526
Sakai S (2003) J Phys Chem A 107:9422
Sakai S (2005) J Mol Struc (THEOCHEM) 715:101
Sakai S (2006) J Phys Chem A 110:6339
Fias S, Damme SV, Bultinck P (2008) J Comput Chem 29:358
Hoffmann MR, Yoshioka Y, Schaefer HF III (1983) J Am Chem Soc 105:1084
Roos B, (1987) In Advances in Chemical Physics; KP Lawley Ed; Wiley: New York, vol. 69, Part II, p 399
Becke AD (1988) Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Nakano H (1993) J Chem Phys 99:7983
Hariharan PC, Pople JA (1973) Theo Chim Acta 28:213
Gordon MS (1980) Chem Phys Lett 76:163
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
Fukui K (1970) J Phys Chem 74:4161
Ishida K, Morokuma K, Komornicki A (1977) J Chem Phys 66:2153
Cundari T, Gordon M (1991) J Am Chem Soc 113:5231
Sakai S (1998) Chem Phys Lett 287:263
Sakai S, Takane S (1999) J Phys Chem A 103:2878
Sakai S (2000) J Phys Chem A 104:922
Sakai S (2002) Int J Quantum Chem 90:549
Boys SF (1960) Rev Mod Phys 32:296
Foster JM, Boys SF (1960) Rev Mod Phys 32:300
Lee PS, Sakai S, Horstermann P, Roth WR, Kallel EA, Houk KN (2003) J Am Chem Soc 125:5839
Sakai S, Nguyen MT (2004) J Phys Chem A 108:9169
Wakayama H, Sakai S (2007) J Phys Chem A 111:13575
Sakai S, Hikida T (2008) J Phys Chem A 112:10985
Yamada T, Udagawa T, Sakai S (2010) Phys Chem Chem Phys 12:3799
Sakai S, Yamada T (2008) Phys Chem Chem Phys 10:3861
Minkin VJ, Glukhovtsev MN, Simkin BY (1994) Aromaticity and antiaromaticity: electronic and structural aspects. Wiley, New York
Schmidt MW, Buldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomerry JA (1993) J Comput Chem 14:1347
Gordon MS, Schmidt MW (2005) Theory and applications of computational chemistry; the first forty years. In: Dykstra CE, Frenking G, Kim KS, Scuseria GE (eds) Elsevier, Amsterdam, pp 1167–1189
Frisch KJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheseman JR, Montgomery JA, Vreven Jr T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox E, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck D, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B (2003) Chen W, Wong MW, Gonzalez C, Pople JA, Gaussian 03; Gaussian, Inc.: Pittsburgh, PA
IDA value of benzene is 0.047 and that of cyclobutadiene with rectangular type is 2.037
Sawicka D, Li Y, Houk KN (1999) J Chem Soc Perkin Trans 2:2349
Acknowledgment
The present research is supported by a Grant-in-Aid for Scientific Research from the Ministry of Education Science and Culture of Japan. The computer time was made available by the Computer Center of the Institute for Molecular Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Akira Imamura on the occasion of his 77th birthday and published as part of the Imamura Festschrift Issue.
Rights and permissions
About this article
Cite this article
Sakai, S., Taketa, K. The [2 + 2 + 2] mechanisms of trimerization of three ethynes and monosilaethylenes. Theor Chem Acc 130, 901–907 (2011). https://doi.org/10.1007/s00214-011-0971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-011-0971-3