Abstract
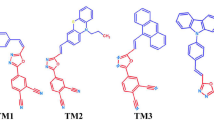
Star-shaped molecules with tailoring functional groups in the core and the arms have great potential application in organic light-emitting devices, because it can be designed to realize low band gap, broad absorption, and excellent solubility for low-cost solution process. To gain an insight into the structure–property relationships, a set of four-arm star-shaped molecules with 2,1,3-benzothiadiazole as the core, different π-conjugated groups as the arm, and triphenylamine or 2-(pyridin-2-yl) pyridine as the end-group were designed. In this study, a systematic investigation into them was carried out using the density functional theory and time-dependent density functional theory methods. The calculated ionization potentials, electron affinities, and reorganization energies (λ) show that the properties of the π-conjugated bridge and the end-group significantly affect the carrier injection and transport characteristics of these molecules, especially for S-BTDP and S-EBTD. Among these molecules, S-BTDP exhibits better electron injection ability due to the introduction of 2-(pyridin-2-yl) pyridine as the end-group. However, S-EBTD, with ethylene as π-conjugated bridge, has excellent hole injection and carrier transport behaviors. We also calculated the singlet-to-triplet exciton-formation cross-section ratio (σS/σT), the exciton-formation fractions (χS), and the absorption and emission spectra of these molecules. We calculated that σS/σT ranges from 1.78 to 2.76 and that χS is ca. 0.37–0.48. These molecules have two absorption bands in the range of 340–410 nm and 500–613 nm, respectively. The calculated emission spectra range from 619 to 706 nm. It can be deduced that the studied 2,1,3-benzothiadiazole-based star-shaped molecules can serve as efficient red light-emitting electroluminescent materials.





Similar content being viewed by others
References
Yang Y, Zhou Y, He QG, He C, Yang CH, Bai FL, Li YF (2009) J Phys Chem B 113:7745
Shirota Y, Kageyama H (2007) Chem Rev 107:953
Tang RP, Tan ZA, Li YF, Xi F (2006) Chem Mater 18:1053
Chen CT (2004) Chem Mater 16:4389
Ren XF, Ren AM, Feng JK, Zhou X (2010) Org Electron 11:979
Wu WC, Yeh HC, Chan LH, Chen CT (2002) Adv Mater 14:1072
Thomas KRJ, Lin JT, Tao YT, Chuen CH (2002) Adv Mater 14:822
Liu J, Shao SY, Chen L, Xie ZY, Cheng YX, Geng YH, Wang LX, Jing XB, Wang FS (2007) Adv Mater 19:1859
Luo J, Li XZ, Hou Q, Peng JB, Yang W, Cao Y (2007) Adv Mater 19:1113
Ma XM, Hua JL, Wu WJ, Jin YH, Meng FS, Zhan WH, Tian H (2008) Tetrahedron 64:345
Du JP, Xu EJ, Zhong HL, Yu F, Liu C, Wu HR, Zeng DL, Ren SJ, Sun J, Liu YC, Cao AM, Fang Q (2008) J Polym Sci Polym Chem Ed 46:1376
Li WW, Du C, Li FH, Zhou Y, Fahlman M, Bo ZS, Zhang FL (2009) Chem Mater 21:5327
He C, He QG, Yi YP, Wu GL, Bai FL, Shuai ZG, Li YF (2008) J Mater Chem 18:4085
Ma CQ, Fonrodona M, Schikora MC, Wienk MM, Janssen RAJ, Bäuerle P (2008) Adv Funct Mater 18:3323
Fischer MKR, Ma CQ, Janssen RAJ, Debaerdemaeker T, Bäuerle P (2009) J Mater Chem 19:4784
Kopidakis N, Mitchell WJ, Lagemaat JVD, Ginley DS, Rumbles G, Shaheen SE, Rance WL (2006) Appl Phys Lett 89:103524
Lee TW, Kim DC, Kang NS, Yu JW, Cho MJ, Kim KH, Choi DH (2008) Chem Lett 37:866
Kim YG, Christian PH, Ananthakrishnan N, Niazimbetova ZI, Thompson BC, Galvin ME, Reynolds JR (2008) Sol Energy Mater Sol Cells 92:307
Alévêque O, Leriche P, Cocherel N, Frère P, Cravino A, Roncali J (2008) Sol Energy Mater Sol Cells 92:1170
Cravino A, Roquet S, Alévêque O, Leriche P, Frère P, Roncali J (2006) Chem Mater 18:2584
Roncali J, Frère P, Blanchard P, Bettignies RD, Turbiez M, Roquet S, Leriche P, Nicolas Y (2006) Thin Solid Films 511–512:567
Zhang XW, Li J, Khan MA, Zhang L, Jiang XY, Khizar-ul H, Zhu WQ, Zhang ZL (2009) Semicond Sci Technol 24:075021
Sousa SF, Fernandes PA, Ramos MJ (2007) J Phys Chem A 111:10439
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian Inc, Wallingford
Chu TY, Ho MH, Chen JF, Chen CH (2005) Chem Phys Lett 415:137
Epstein AJ, Lee WP, Prigodin VN (2001) Synth Met 117:9
Reedijk JA, Marten HCF, van Bohemena SMC, Hilt O, Brom HB, Michelsb MAJ (1999) J Synth Met 101:475
Mott NF, Davis EA (1979) Electronic processes in non-crystalline materials, 2nd edn. Oxford University Press, Oxford
Hutchison GR, Ratner MA, Marks TJ (2005) J Am Chem Soc 127:2339
Marcus RA (1993) Rev Mod Phys 65:599
Marcus RA, Eyring H (1964) Annu Rev Phys Chem 15:155
Hush NS (1958) J Chem Phys 28:962
Marcus RA (1956) J Chem Phys 24:966
Chernyak V, Meier T, Tsiper E, Mukamel S (1999) J Phys Chem A 103:10294
Barzilai IL, Bulatov V, Schechter I (2004) Anal Chim Acta 501:151
Lukeš V, Aquino A, Lischka H (2005) J Phys Chem A 109:10232
Zou LY, Ren AM, Feng JK, Ran XQ (2009) J Phys Org Chem 22:1104
Wohlgenannt M, Kunj T, Mazumdar S, Ramasesha S, Vardeny ZV (2001) Nature 409:494
Karabunarliev S, Bittner ER (2003) Phys Rev Lett 90:057402
Wohlgenannt M, Jiang XM, Vardeny ZV, Janssen RAJ (2002) Phys Rev Lett 88:197401
Baldo MA, O’Brien DF, Thompson ME, Forrest S (1999) Phys Rev B 60:14422
Ho PKH, Kim JS, Burroughes JH, Becker H, Li SFY, Brown TM, Cacialli F, Friend RH (2000) Nature 404:481
Cao Y, Parker ID, Yu G, Zhang C, Heeger AJ (1999) Nature 397:414
Wilson JS, Dhoot AS, Seeley AJAB, Khan MS, Köhler A, Friend RH (2001) Nature 413:828
Shuai Z, Beljonne D, Silbey RJ, Brédas JL (2000) Phys Rev Lett 84:131
Chen LP, Zhu LY, Shuai ZG (2006) J Phys Chem A 110:13349
Yin SW, Chen LP, Xuan PF, Chen KQ, Shuai Z (2004) J Phys Chem B 108:9608
Yin J, Chen RF, Zhang SL, Ling QD, Huang W (2010) J Phys Chem A 114:3655
Acknowledgments
This work is supported by the Major State Basic Research Development Program (2002CB 613406), the National Natural Science Foundation of China (Project No. 20973078), the State Key Laboratory of Theoretical and Computational Chemistry of Jilin University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YF., Ren, XF., Zou, LY. et al. Theoretical study on photophysical properties of 2,1,3-benzothiadiazole-based star-shaped molecules. Theor Chem Acc 129, 833–845 (2011). https://doi.org/10.1007/s00214-011-0942-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-011-0942-8