Abstract
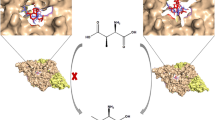
The enzyme aspartate racemase from Pyrococcus horikoshii OT3 catalyzes the interconversion between l- and d-Asp. In this work, we employed the hybrid QM/MM approach with the self-consistent charge-density functional tight binding (SCC-DFTB) model to study the catalytic mechanism for the conversion of l-Asp into d-Asp. The molecular dynamics simulation showed that the substrate l-Asp forms an extensive network of interactions with the active-site residues of the aspartate racemase through its side chain carboxylate, ammonium group, and α-carboxylate. The potential of mean force calculations confirmed that the racemization reaction involves two proton transfers (from the α-carbon to Cys194 and from Cys82 to the α-carbon), which occurs in a concerted way, although highly asynchronous. The calculated free energy of activation is 17.5 kcal/mol, which is consistent with the reaction rate measured from experiment. An electrostatic interaction analysis was performed to estimate the key role played by individual residues in stabilizing the transition state. The docking study on the binding of l-Asp and d-Asp to aspartate racemase indicates that this enzyme employs a “two-base” mechanism not a “one-base” mechanism.










Similar content being viewed by others
References
Walsh CT (1989) J Biol Chem 264:2393–2396
Yohda M, Endo I, Abe Y, Ohta T, Iida T, Maruyama T, Kagawa Y (1996) J Biol Chem 271:22017–22021
Matsumoto M, Homma H, Long Z, Imai K, Iida T, Maruyama T, Aikawa Y, Endo I, Yohda M (1999) J Bacteriol 181:6560–6563
Dunlop DS, Neidle A, AcHale D, Dunlop DM, Lajtha A (1986) Biochem Biophys Res Commun 141:27–32
Wolosker H, D’Aniello A, Synder SH (2000) Neuroscience 100:183–189
Johnston MM, Diven WF (1969) J Biol Chem 244:5414–5420
Gerlt JA, Kenyon GL, Kozarich JW, Neidhart DJ, Petsko GA, Powers VM (1992) Curr Opin Struct Biol 2:736–742
Hayashi H, Wada H, Yoshimura T, Esaki N, Soda K (1990) Annu Rev Biochem 59:87–110
Nakajima N, Tanizawa K, Tanaka H, Soda K (1986) Agric Biol Chem 50:2823–2830
Albery WJ, Knowles JR (1986) Biochemistry 25:2572–2577
Higgins W, Tardif C, Richaud C, Krivanek MA, Cardin A (1989) Eur J Biochem 186:137–143
Finlay TH, Adams EJ (1970) J Biol Chem 245:5248–5260
Gallo KA, Tanner ME, Knowles JR (1993) Biochemistry 32:3991–3997
Tanner ME, Gallo KA, Knowles JR (1993) Biochemistry 32:3998–4006
Koo CW, Blanchard JS (1999) Biochemistry 38:4416–4422
Fisher LM, Belasco JG, Bruice TW, Albery WJ, Knowles JR (1986) Biochemistry 25:2543–2551
Yamauchi T, Choi SY, Okada H, Yohda M, Kumagai H, Esaki N, Soda K (1992) J Biol Chem 267:18361–18364
Liu L, Iwata K, Kita A, Kawarabayasi Y, Yohda M, Miki K (2002) J Mol Biol 319:479–489
Yoshida T, Seko T, Okada O, Iwata K, Liu L, Miki K, Yohda M (2006) Proteins 64:502–512
Ohtaki A, Nakano Y, Iizuka R, Arakawa T, Yamada K, Odaka M, Yohda M (2008) Proteins 70:1167–1174
Puig E, Garcia-Viloca M, Gonzalez-Lafont A, Lluch JM, Field MJ (2007) J Phys Chem B 111:2385–2397
Puig E, Mixcoha E, Garcia-Viloca M, Gonzalez-Lafont A, Lluch JM (2009) J Am Chem Soc 131:3509–3521
Spies MA, Reese JG, Dodd D, Pankow KL, Blanke SR, Baudry J (2009) J Am Chem Soc 131:5274–5284
Stenta M, Calvaresi M, Alto P, Spinelli D, Garavelli M, Bottoni A (2008) J Phys Chem B 112:1057–1059
Rubinstein A, Major DT (2009) J Am Chem Soc 131:8513–8521
Stenta M, Calvaresi M, Altoe P, Spinelli D, Garavelli M, Galeazzi R, Bottoni A (2009) J Chem Theory Comput 5:1915–1930
Field MJ, Bash PA, Karplus M (1990) J Comput Chem 11:700–733
Gao J (1996) Acc Chem Res 29:298–305
Warshel A (2003) Annu Rev Biophys Biomol Struct 32:425–443
Riccardi D, Schaefer P, Yang Y, Yu H, Ghosh N, Prat-Resina X, Konig P, Li G, Xu D, Guo H, Elstner M, Cui Q (2006) J Phys Chem B 110:6458–6469
Friesner RA, Guallar V (2005) Annu Rev Phys Chem 56:389–427
Elstner M, Porezag D, Jungnickel G, Elsner J, Haugk M, Frauenheim T, Suhai S, Seigert G (1998) Phys Rev B58:7260–7268
Cui Q, Elstner M, Kaxiras E, Frauenheim T, Karplus M (2001) J Phys Chem B 105:569–585
Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E (2001) J Chem Phys 114:5149–5155
Pu J, Gao J, Truhlar DG (2004) J Phys Chem A 108:5454–5463
Witek HA, Morokuma K (2004) J Comput Chem 25:1858–1864
Cui Q, Elstner M, Karplus M (2002) J Phys Chem B 106:2721–2740
Zhang X, Harrison DH, Cui Q (2002) J Am Chem Soc 124:14871–14878
Guo H, Rao N, Xu Q, Guo H (2005) J Am Chem Soc 127:3191–3197
Liu J, Wang X, Xu D (2010) J Phys Chem B 114:1462–1470
Xu Q, Li L, Guo H (2010) J Phys Chem B 114:10594–10600
MacKerell AD Jr, Bashford D, Bellott M, Dunbrack RL Jr, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE III, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) J Phys Chem B 102:3586–3616
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) J Comput Chem 4:187–217
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79:926–935
Brooks CL III, Brunger A, Karplus M (1985) Biopolymers 24:843–865
Steinbach PJ, Brooks BR (1994) J Comput Chem 15:667–683
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) J Comput Phys 23:327–341
Torrie GM, Valleau JP (1977) J Comput Phys 23:187–199
Kumar S, Bouzida D, Swendsen RH, Kollman PA, Rosenberg JM (1992) J Comput Chem 13:1011–1021
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) J Comput Chem 19:1639–1662
National Center for Biotechnology Information. PubChem Compound Database (2011) CID = 5460294, http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5460294&loc=ec_rcs accessed Mar 11 2011
National Center for Biotechnology Information. PubChem Compound Database (2011); CID = 5460295, http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5460295&loc=ec_rcs accessed Mar 11 2011
Schuettelkopf AW, van Aalten DMF (2004) Acta Cryst D 60:1355–1363
Weiner SJ, Kollman PA, Case DA, Singh UC, Ghio C, Alagona G, Profeta S, Weiner P (1984) J Am Chem Soc 106:765–784
Elstner M, Frauenheim T, Kaxiras E, Seifert G, Suhai S (2000) Phys Status Solidi B 217:357–376
Riccardi D, Konig P, Guo H, Cui Q (2008) Biochemistry 47:2369–2378
Xu Q, Guo H, Gorin A, Guo H (2007) J Phys Chem B 111:6501–6506
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Acknowledgments
This project has been supported by the National Natural Science Foundation of China (Grant Nos. 20773089 and 21075083) and National Basic Research Program of China (973 Program) (2011CB201202). We appreciate Prof. Dingguo Xu for his many stimulating discussions. The CHARMM calculations have been carried out in Wuhan Institute of Physics and Mathematics, the Chinese Academy of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Guo, Y. & Xue, Y. QM/MM study on catalytic mechanism of aspartate racemase from Pyrococcus horikoshii OT3. Theor Chem Acc 129, 781–791 (2011). https://doi.org/10.1007/s00214-011-0935-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-011-0935-7