Abstract
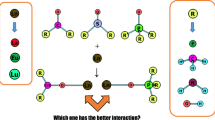
Novel comparison of the structural, electronic and energy aspects of lanthanide complexes of model phosphoramides (PAs) with those of phosphine oxides (POs), phosphate esters (PEs) and phosphoryl trihalides (PHs) has been carried out by ab initio and DFT calculations. Atoms in Molecules (AIM) and Natural Bonding Orbital (NBO) analyses were performed to understand the electronic structure of ligands L and related complexes, L–Ln3+. NBO analysis indicates that the negative charge on phosphoryl oxygen (OP) and the p character of the phosphoryl lone pair, Lp(OP), increase in the order PH < PE < PO < PA. Positive charge of the lanthanide cation in PA complexes is less than those of PH, PE and PO complexes, due to the more intense ligand to metal charge transfer (LMCT). The metal–ligand distance decreases in the order PH > PE > PO > PA, which is confirmed by the results of AIM analysis. Charge density at the bond critical point of L–Ln3+ follows the sequence PH < PE < PO < PA. The results of the Energy Decomposition Analysis (EDA) indicate that the donative interaction and LMCT increases in order PH < PO < PE < PA. The effect of basis set superposition error (BSSE) on the L···Ln3+ interaction energies was also studied in detail at DFT, MP2 and CCSD(T) levels using the counterpoise (CP) method. Trends in the CP-corrected L–Ln3+ bond energies are in good accordance with the optimized OP···Ln3+ distances. The results show that the difference between CP-corrected and uncorrected interaction energies in PA complexes is larger than those in the others, because PAs are more deformable. It is depicted that PAs are comparable with POs in lanthanide complexation.

Similar content being viewed by others
References
Horwitz EP, Kalina DG, Diamond H, Vandegrift GF, Schulz WW (1985) Solvent Extr Ion Exch 3:75–109
Nash KL (1993) Solvent Extr Ion Exch 11:729–768
Bhattacharyya A, Mohapatra PK, Manchanda VK (2006) Solvent Extr Ion Exch 24:1–17
Modolo G, Nabet S (2005) Solvent Extr Ion Exch 23:359–373
Pierce TB, Peck PF (1963) Analyst 88:217–221
Yaftian MR, Burgard M, Matt D, Dieleman CB, Rastegar F (1997) Solvent Extr Ion Exch 15(6):975–989
Boehme C, Wipff G (2001) Chem Eur J 7:1398–1407
Nazarenko AY, Baulin VE, Lamb JD, Volkova TA, Varnek AA, Wipff G (1999) Solvent Extr Ion Exch 17(3):495–523
Atamas L, Klimchuk O, Rudzevich V, Pirozhenko V, Kalchenko V, Smirnov I, Babain V, Efremova T, Varnek A, Wipff G, Arnaud-Neu F, Roch M, Saadioui M, Bohmer V (2002) J Supramol Chem 2:421–427
Jenkins AL, Uy OM, Murray GM (1999) Anal Chem 71:373–378
Alexander V (1995) Chem Rev 95:273–342
Dam HH, Beijleveld H, Reinhoudt DN, Verboom W (2008) J Am Chem Soc 130:5542–5551
Berny F, Muzet N, Troxler L, Dedieu A, Wipff G (1999) Inorg Chem 38:1244–1252
Baaden M, Berny F, Boehme C, Muzet N, Schurhammer R, Wipff G (2000) J Alloys Compd 303–304:104–111
Schurhammer R, Erhart V, Troxler L, Wipff G (1999) J Chem Soc Perkin Trans 2:2423–2431
Troxler L, Hutschka DF, Wipff G (1998) J Mol Struct: THEOCHEM 431:151–163
Troxler L, Baaden M, Bohmer V, Wipff G (2000) Supramol Chem 12:27–51
Boehme C, Wipff G (2002) Inorg Chem 41:727–737
Chu IH, Zhang H, Dearden DV (1993) J Am Chem Soc 115:5736–5744
Staley RH, Beauchamp JL (1975) J Am Chem Soc 97:5920–5921
Hutschka F, Dedieu A, Troxler L, Wipff G (1998) J Phys Chem A 102:3773–3781
Pearson RG (1990) Coord Chem Rev 100:403–425
Hancock RD, Martell AE (1989) Chem Rev 89:1875–1914
Berny F, Wipff G (2001) J Chem Soc Perkin Trans 2:73–82
Modolo G, Odoj R (1999) Solvent Extr Ion Exch 17:33–53
Corbridge DEC (1995) Phosphorus: an outline of its chemistry, biochemistry and technology, 5th edn. Elsevier, Amsterdam
Wiberg E, Wiberg N, Holleman AF (2001) Inorganic chemistry. Academic Press, London, pp 613–614
Maron L, Eisenstein O (2000) J Phys Chem A 104:7140–7143
Cotton S (2006) Lanthanide and actinide chemistry. Wiley, Chichester
Dolg M, Stoll H, Savin A, Preuss H (1989) Theor Chim Acta 75:173–194
Dolg M, Stoll H, Savin A, Preuss H (1993) Theor Chim Acta 85:441–450
Ehlers AW, Bohme M, Dapprich S, Gobbi A, Hollwarth A, Jonas V, Kohler KF, Stegmann R, Veldkamp A, Frenking G (1993) Chem Phys Lett 208:111–114
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Bader RFW (1990) Atoms in molecules: A quantum theory. Oxford University Press, Oxford, UK
Bader RFW (1991) Chem Rev 91:893–928
Matta CF, Boyd RJ (2007) The Quantum Theory of Atoms in Molecules. WILEY-VCH Verlag GmbH & Co, KGaA, Weinheim
Rotzinger FP (2005) J Phys Chem B 109:1510–1527
Wahlin P, Danilo C, Vallet V, Real F, Flament JP, Wahlgren U (2008) J Chem Theory Comput 4:569–577
Torrent M, Gili P, Duran M, Sola M (1996) J Chem Phys 104:9499–9510
Dunbar RC (2002) J Phys Chem A 106:7328–7337
Fan HJ, Liu CW (1999) Chem Phys Lett 300:351–358
Chalasinski G, Szczesniak MM (1994) Chem Rev 94:1723–1765
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, revision A.7. Gaussian, Inc. Pittsburgh, PA
Kitaura K, Morokuma K (1976) Int J Quantum Chem 10:325–340
Morokuma K, Kitaura K (1981) Chemical applications of atomic and molecular electrostatic potentials. In: Politzer P, Truhlar DG (Eds). Plenum, New York
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Gilheany DG (1994) Chem Rev 94:1339–1374
Dobado JA, Martinez-Garcia H, Molina JM, Sundberg MR (1998) J Am Chem Soc 120:8461–8471
Bollinger JC, Houriet R, Kern CW, Perret D, Weber J, Yvernault T (1985) J Am Chem Soc 107:5352–5358
Xantheas SS (1996) J Chem Phys 104:8821–8824
Jensen HB, Ross P (1969) Chem Phys Lett 3:140–143
Liu B, McLean AD (1973) J Chem Phys 59:4557–4558
Kim CK, Zhang H, Yoon SH, Won J, Lee MJ, Kim CK (2009) J Phys Chem A 113:513–519
Coupez B, Boehme C, Wipff G (2002) Phys Chem Chem Phys 4:5716–5729
Acknowledgment
Support of this work by Tarbiat Modares University is gratefully acknowledged. We thank also Dr. Afshin Abbasi for his comments and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gholivand, K., Mahzouni, H.R. & Esrafili, M.D. How do phosphoramides compete with phosphine oxides in lanthanide complexation? Structural, electronic and energy aspects at ab initio and DFT levels. Theor Chem Acc 127, 539–550 (2010). https://doi.org/10.1007/s00214-010-0743-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-010-0743-5