Abstract
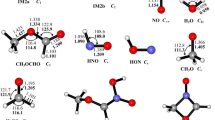
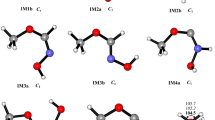
The mechanism of the gas-phase reaction OH with CH2=C(CH3)CH2OH (2-methyl-2-propen-1-ol) has been elucidated using high-level ab initio method, i.e., CCSD(T)/6-311++g(d,p)//MP2(full)/6-311++g(d,p). Various possible H-abstraction and addition–elimination pathways are identified. The calculations indicate that the addition–elimination mechanism dominates the OH+MPO221 reaction. The addition reactions between OH radicals and CH2=C(CH3)CH2OH begin with the barrierless formation of a pre-reactive complex in the entrance channel, and subsequently the CH2(OH)C(CH3)CH2OH (IM1) and the CH2C(OH)(CH3)CH2OH (IM2) are formed by OH radicals’ electrophilic additions to the double bond. IM1 can easily rearrange to IM2 via a 1,2-OH migration. Subsequently, rearrangement of IM2 to form (CH3)2C(OH)CH2O (IM11) followed by dissociation to HCHO + (CH3)2COH (P21) is the most favorable pathway. The decomposition of IM2 to CH2OH + CH2=C(OH)CH3 (P16) is the secondary pathway. The other pathways are not expected to play any important role in forming final products.







Similar content being viewed by others
References
König G, Brunda M, Puxbaum H, Hewitt CN, Duckham SC, Rudolph J (1995) Atmos Environ 29:861
Cometto PM, Dalmasso PR, Taccone RA, Lane SI, Oussar F, Daële V, Mellouki A, Bras GL (2008) J Phys Chem A 112:4444
Guenther AB, Hewitt CN, Erickson D, Fall R, Geron C, Graedel R, Harley P, Klinger L, Lerdau M, Mckay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P (1995) J Geophys Res 100:8873
Imamura T, Iida Y, Obi K, Nagatani I, Nakagawa K, Patroescu-Klotz I, Hatakeyama S (2004) Int J Chem Kinet 36:379
Papagni C, Arey J, Atkinson R (2001) Int J Chem Kinet 33:142
Grosjean D, Grosjean E, Williams EL II (1993) Environ Sci Technol 27:2478
Orlando JJ, Tyndall GS, Ceazan N (2001) J Phys Chem A 105:3564
Rudich Y, Talukdar R, Burkholder JB, Ravishankara AR (1995) J Phys Chem 99:12188
Hallquist M, Langer S, Ljungström E, Wängberg I (1996) Int J Chem Kinet 28:467
Noda J, Nyman G, Langer S (2002) J Phys Chem A106:945
Rodriguez D, Rodriguez A, Soto A, Aranda A, Diaz-de-Mera Y, Notario A (2008) J Atmos Chem 59:187
Rodriguez A, Rodriguez D, Soto A, Notario A, Aranda A, Diaz-de-Mera Y, Bravo I (2007) Atmos Environ 41:4693
Grosjean D, Grosjean E, Williams EL II (1993) Int J Chem Kinet 25:783
Grosjean E, Grosjean D (1994) Int J Chem Kinet 26:1185
Frisch MJ et al (2004) Gaussian 03, Revision C.02. Gaussian, Inc., Wallingford
Møller C, Plesset MS (1934) Phys Rev 46:618
Fletcher GD, Rendell AP, Sherwood P (1997) Mol Phys 91:431
Hehre WJ, Radom L, Pople JA, Schleyer PR (1987) Ab initio molecular orbital theory. Wiley, New York
EMSL Basis Set Library. http://www.emsl.pnl.gov/forms/basisform.html
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Garrett BC, Redmon MJ, Steckler R, Truhlar DG, Baldridge KK, Bartol D, Schmidt MW, Gordon MS (1988) J Phys Chem 92:1476
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) Chem Phys Lett 157:479
Werner HJ, Knowles PJ (1985) J Chem Phys 82:5053
Knowles PJ, Werner HJ (1985) Chem Phys Lett 115:259
Jalbout AF, Boutalib A (2006) J Phys Chem A 110:12524
Acknowledgments
This work is supported by the Natural Science Foundation of Xuzhou Normal University (07XLA05). The authors express their gratitude to the referees for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, W., Du, B. & Feng, C. Theoretical investigation on mechanism for OH-initiated oxidation of CH2=C(CH3)CH2OH. Theor Chem Acc 125, 45–55 (2010). https://doi.org/10.1007/s00214-009-0657-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0657-2