Abstract
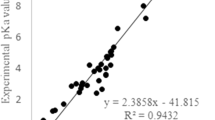
1H and 13C NMR chemical shifts of coumarin derivatives have been determined using first principles approaches with and without accounting for the effects of the solvent and compared to experiment in order to assess their reliability. Good linear relationships are obtained between theory and experiment, which allows correcting the calculated values for systematic errors. This is particularly the case when using the PCM scheme to model the solvent effects because the δ values larger than 150 ppm are more difficult to reproduce. The final accuracy of the method amounts to about 1 ppm for 13C and 0.05 ppm for 1H.



Similar content being viewed by others
References
Helgaker T, Jaszunski M, Ruud K (1999) Chem Rev 99:293
Gauss J, Stanton JF (2002) Adv Chem Phys 123:355
Kaupp M, Bühl M, Malkin VG (eds) (2004) Calculation of NMR and EPR parameters. Wiley-VCH, Weinheim
Bagno A, Rastrelli F, Saielli G (2006) Chem Eur J 12:5514
Bifulco G, Dambruoso P, Gomez-Paloma L, Riccio R (2007) Chem Rev 107:3744
Ando I, Kuroki S, Kurosu H, Yamanobe T (2001) Prog NMR Spectrosc 39:79
d’Antuono Ph, Botek E, Champagne B, Wieme J, Reyniers M–F, Marin GB, Adriaensens PJ, Gelan JM (2007) Chem Phys Lett 436:388
Chesnut DB (1996) The ab initio computation of nuclear magnetic resonance chemical shielding. In: Lipkowitz KB, Boyd DB (eds) Reviews in computational chemistry, vol 8. VCH, Weinheim, p 245
Rablen PR, Pearlman SA, Finkbiner J (1999) J Phys Chem A 103:7357
d’Antuono Ph, Botek E, Champagne B, Spassova M, Denkova P (2006) J Chem Phys 125:144309
Bifulco G, Gomez-Paloma L, Riccio R (2003) Tetrahedron Lett 44:7137
Trabelsi M, Salem M, Champagne B (2003) Org Biomol Chem 1:3839
Poater J, van Lenthe E, Baerends EJ (2003) J Chem Phys 118:8584
Allen MJ, Keal TW, Tozer DJ (2003) Chem Phys Lett 380:380
Tähtinen P, Bagno A, Klika KD, Pihlaja K (2003) J Am Chem Soc 125:4609
Hieringer W, Della Sala F, Görling A (2004) Chem Phys Lett 383:115
Arbuznikov AV, Kaupp M (2004) Chem Phys Lett 386:8
Gryffer-Keller A, Molchanov S (2004) Mol Phys 102:1903
Wiitala KW, Hoye TR, Cramer CJ (2006) J Chem Theor Comput 2:1085
Wiitala KW, Al-Rashid ZF, Dvornikovs V, Hoye TR, Cramer CJ (2007) J Phys Org Chem 20:345
Wiitala KW, Cramer CJ, Hoye TR (2007) Magn Reson Chem 45:819
Rychnovsky SD (2006) Org Lett 8:2895
Bagno A, Rastrelli F, Saielli G (2007) J Org Chem 72:7373
Pennanen TS, Lantto P, Sillanpää AJ, Vaara J (2007) J Phys Chem A 111:182
Bassarello C, Bifulco G, Montoro P, Skhirtladze A, Kemertelidze E, Pizza C, Piacente S (2007) Tetrahedron 63:148
Baldridge KK, Siegel JS (2008) Theor Chem Acc 120:95
Habib-Jiwan J-L, Branger C, Soumillon J-Ph, Valeur B (1998) J Photochem Photobiol A Chem 116:127
Leray I, Habib-Jiwan J-L, Branger C, Soumillon JPh, Valeur B (2000) J Photochem Photobiol A Chem 135:163
Taziaux D, Soumillon J-Ph, Habib-Jiwan J-L (2004) J Photochem Photobiol A Chem 162:599
Maton L, Taziaux D, Soumillion J-Ph, Habib Jiwan J-L (2005) J Mater Chem 15:2928
Löhr HG, Vögtle F (1985) Acc Chem Res 18:65
Valeur B (2001) Molecular fluorescence: principles and applications. Wiley-VCH Verlag GmbH, Weinheim
Fedorova OA, Fedorov YV, Vedernikov AI, Gromov SP, Yescheulova OV, Alfimov MV, Woerner M, Bossmann S, Braun A, Saltiel J (2002) J Phys Chem A 106:6213
Korolev VV, Vorobyev DYu, Glebov EM, Grivin VP, Plyusnin VF, Koshkin AV, Fedorova OA, Gromov SP, Alfimov MV, Shklyaev YV, Vshivkova TS, Rozhkova YS, Tolstikov AG, Lokshin VV, Samat A (2007) J Photochem Photobiol A Chem 192:75
Berthet J, Micheau JC, Lokshin V, Vales M, Vermeersch G, Delbaere S (2008) Org Lett 10:3773
Ditchfield R (1971) J Am Chem Soc 93:5287
Wolinski K, Hilton JF, Pulay P (1990) J Am Chem Soc 112:8251
Cammi R, Mennucci B, Tomasi J (1999) J Chem Phys 110:7627
Manalo MN, de Dios AC, Cammi R (2000) J Phys Chem A 104:9600
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) GAUSSIAN 03, Revision C.02. Gaussian, Inc., Wallingford
Filatov M, Cremer D (2005) J Chem Phys 123:124101
d’Antuono Ph, Botek E, Champagne B, Wieme J, Reyniers M–F, Marin GB, Adriaensens PJ, Gelan JM (2005) Chem Phys Lett 211:207
d’Antuono Ph, Botek E, Champagne B, Wieme J, Reyniers M-F, Marin GB, Adriaensens PJ, Gelan JM (2008) J Phys Chem B 112:14804
Acknowledgments
This work is dedicated to Prof. S. Suhai, a leading scientist in the field of polymer electronic structure calculations. This work was supported from research grants from the Belgian Government (IUAP No P06-27 “Functional Supramolecular Systems”). Ph. d’A. is grateful to the Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA) for his PhD grant. E.B. thanks the IUAP program No P06-27 for her postdoctoral grants. B.C. thanks the Belgian National Fund for Scientific Research for his Research Director position and L.M. for her research fellow position. The calculations were performed on the Interuniversity Scientific Computing Facility (ISCF), installed at the Facultés Universitaires Notre-Dame de la Paix (Namur, Belgium), for which the authors gratefully acknowledge the financial support of the FNRS-FRFC, and the “Loterie Nationale” for the convention no 2.4.617.07.F, and of the FUNDP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Sandor Suhai on the occasion of his 65th birthday and published as part of the Suhai Festschrift Issue.
Rights and permissions
About this article
Cite this article
d’Antuono, P., Botek, E., Champagne, B. et al. A joint theoretical and experimental investigation on the 13C and 1H NMR chemical shifts of coumarin derivatives. Theor Chem Acc 125, 461–470 (2010). https://doi.org/10.1007/s00214-009-0625-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0625-x