Abstract
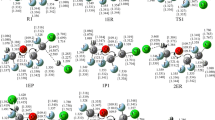
Theoretical investigations are carried out on the reaction Cl + CH2FCl by means of direct dynamics method. The minimum energy path (MEP) is obtained at the MP2/6-311G(d, p) level. The energetic information is further improved by single-point energy calculations using QCISD(T)/6-311++G(d, p) method. The kinetics of this reaction are calculated by canonical variational transition state theory incorporating with the small-curvature tunneling correction over a wide temperature range of 220–3,000 K, and rate constant expression are found to be k(T) = 1.48 × 10−17 T 2.04exp(−913.91/T). For the title reaction, H-abstraction reaction channel is the major channel at the lower temperatures. At higher temperatures, the contribution of Cl-abstraction reaction channel should be taken into account.





Similar content being viewed by others
References
Tuck R, Plumb A, Condon E (1990) Geophys Res Lett 17:313. doi:10.1029/GL017i004p00313
Rosswall T (1991) Environ Sci Technol 25:567. doi:10.1021/es00016a001
Atkinson R (1986) Chem Rev 86:69. doi:10.1021/cr00071a004
Atkinson R (1994) J Phys Chem Ref Data Monograph 2:1–216
Manzer L (1990) Science 249:31. doi:10.1126/science.249.4964.31
Manning RG, Kurylo MJ (1977) J Phys Chem 81:291. doi:10.1021/j100519a003
Senkan SM (1988) Environ Sci Technol 22:368. doi:10.1021/es00169a600
Bryukov MG, Slagle IR, Knyazev VD (2001) J Phys Chem A 105:6900. doi:10.1021/jp0106505
Zhang H, Zhang GL, Wang L, Liu B, Yu XY, Li ZS (2006) Chem Phys Lett 432:6. doi:10.1016/j.cplett.2006.10.024
Knyazev VD (2003) J Phys Chem A 107:11082. doi:10.1021/jp036281p
Li S, Li ZS, Liu JY, Sun CC (2004) J Comput Chem 25:72. doi:10.1002/jcc.10305
Li QS, Wang CY (2002) Phys Chem Chem Phys 4:4386. doi:10.1039/b206133n
Sun H, He HQ, Pan YR, Pan XM, Li ZS, Wang RS (2008) Chem Phys Lett 450:186. doi:10.1016/j.cplett.2007.11.003
Zhang H, Wu JY, Li ZS, Liu JY, Li S, Sun CC (2005) J Comput Chem 26:1421. doi:10.1002/jcc.20283
Wang L, Liu JY, Wan SQ, Li ZS (2008) Chem Phys Lett 455:20. doi:10.1016/j.cplett.2008.02.006
Tschuikow-Roux E, Faraji F, Paddison S, Niedzielski J, Miyokawa K (1988) J Phys Chem 92:1488. doi:10.1021/j100317a023
Jourdain GL, Poulet G, Barassin J, LeBras G, Combourieu J (1977) J Pollut Atmos 75:256
Tuazon EC, Atkinson R, Corchnoy SB (1992) Int J Chem Kinet 24:639. doi:10.1002/kin.550240704
Senkan SM, Quam D (1992) J Phys Chem 96:10837. doi:10.1021/j100205a044
Wallington TJ, Hurley MD, Schneider WF, Sehested J, Nielsen OJ (1994) Chem Phys Lett 218:34. doi:10.1016/0009-2614(93)E1466-T
Atkinson R, Baulch DL, Cox RA et al (1997) J Phys Chem Ref Data 26:521
Bell RL, Truong TN (1994) J Chem Phys 101:10442. doi:10.1063/1.467862
Truong TN, Duncan WT, Bell RL (1996) In: Chemical applications of density functional theory. American Chemical Society, Washington, DC, p 85
Truhlar DG (1995) In: Heidrich D (ed) The reaction path in chemistry: current approaches and perspectives. Kluwer, Dordrecht, p 229
Corchado JC, Espinosa-Garcia J, Hu WP, Rossi I, Truhlar DG (1995) J Phys Chem 99:687. doi:10.1021/j100002a037
Hu WP, Truhlar DG (1996) J Am Chem Soc 118:860. doi:10.1021/ja952464g
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440. doi:10.1021/ar50156a002
Truhlar DG, Isaacson AD, Garrett BC (1985) In: Baer M (ed) The theory of chemical reaction dynamics, vol 4. CRC Press, Boca Raton, p 65
Frisch MJ, Trucks GW, Schlegel HB et al (2004) Gaussian 03, revision C.02. Gaussian, Inc., Wallingford
Duncan WT, Truong TN (1995) J Chem Phys 103:9642. doi:10.1063/1.470731
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154. doi:10.1063/1.456010
Corchado JC, Chuang YY, Fast PL et al (2002) POLYRATE version 9.1. Department of Chemistry and Supercomputer Institute, University of Minnesota, Minneapolis, MN
Chuang YY, Corchado JC, Truhlar DG (1990) J Phys Chem A 103:1140. doi:10.1021/jp9842493
Garrett BC, Truhlar DG (1979) J Chem Phys 70:1593. doi:10.1063/1.437698
Garrett BC, Truhlar DG (1979) J Am Chem Soc 101:4534. doi:10.1021/ja00510a019
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730. doi:10.1021/j100450a013
Truhlar DG, Issacson AD, Skodje RT, Garrett BC (1983) J Phys Chem 87:4554. doi:10.1021/j100245a604
Lu DH et al (1992) Comput Phys Commun 71:235. doi:10.1016/0010-4655(92)90012-N
Liu YP, Lynch GC, Truong TN, Lu DH, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408. doi:10.1021/ja00059a041
Lide DR (1999) In: CRC Handbook of chemistry and physics, 80th edn. CRC Press, Boca Raton
Huber KP, Herzberg G (1979) In: Molecular spectra and molecular structure IV. Constants of diatomic molecules. Van Nostrand Reinhold Co., New York
Harmony MP, Laurie VW, Ramsay RL, Lovas FJ, Lafferty WJ et al (1979) J Phys Chem Ref Data 8:619
Chase MW Jr, Davies CA, Downey JR, Fryrip DJ, McDonald RA, Syverud AN JANAF (1985) Thermochemical tables, Suppl 1, vol 14, 3rd edn, Ref Data, pp 1–926
NIST Chemistry WebBook (2005) NIST Standard Reference Database Number 69, June Release (vibrational frequency data compiled by Jacox ME)
NIST Chemistry WebBook (2003) NIST Standard Reference Database Number 69, March Release (date compiled by Huber KP and Herzberg G)
Chase MW Jr (1998) Thermochemical tables NIST-JANAF, 4th edn. J Phys Chem Ref Data, Monograph 9, Suppl 1
Tschuikow-Roux E, Paddison S (1987) Int J Chem Kinet 19:15. doi:10.1002/kin.550190103
Poutsma JC, Paulino JA, Squires RR (1997) J Phys Chem A 101:5327. doi:10.1021/jp970778f
Rosenman E, McKee ML (1997) J Am Chem Soc 119:9033. doi:10.1021/ja971185l
Xiao JF, Li ZS, Ding YH et al (2002) J Phys Chem A 106:320. doi:10.1021/jp013405u
Acknowledgments
The authors thank Prof. Donald G. Truhlar for providing POLYRATE 9.1 program. This work is supported by the National Natural Science Foundation of China (No. 20773021), and Training Fund of NENU’S Scientific Innovation Project (NENU-STC07016). We are greatly thankful for the referees’ helpful comments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jia, XJ., Liu, YJ., Sun, JY. et al. Mechanistic and dual-level direct dynamics studies on the reaction Cl + CH2FCl. Theor Chem Acc 124, 105–113 (2009). https://doi.org/10.1007/s00214-009-0587-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0587-z