Abstract
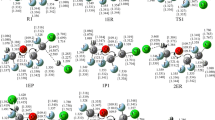
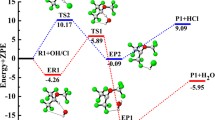
The hydrogen abstraction reactions of CF3CF2CFH2 and CF3CFHCF2H with OH radicals and Cl atoms have been studied theoretically by a dual-level direct dynamics method. Two stable conformers of CF3CF2CFH2 with C s and C 1 symmetries and all possible abstraction channels for each reaction are all taken into consideration. Optimized geometries and frequencies of all the stationary points and extra points along minimum-energy path (MEP) have been computed at the BB1K/6-31+G(d, p) level of theory. To refine the energy profile of each reaction channel, single point energy calculations have been performed by the BMC-CCSD method. The rate constants are evaluated by canonical variational transition state theory (CVT) with the small-curvature tunneling correction method (SCT) over a wide temperature range of 200–1,000 K. The detailed branching ratios of four reactions are discussed. The good agreement found between our theoretical rate constants and the available experimental data suggests that the present approach could provide a reliable prediction for the CF3CFHCF2H + Cl reaction about which there is little experimental information. The kinetic calculations show that the SCT effect plays an important role in all channels. In addition, in order to further reveal the thermodynamic properties, the enthalpies of formation of the reactants (CF3CF2CFH2 and CF3CFHCF2H) and the product radicals (CF3CF2CFH, CF3CFCF2H, and CF3CFHCF2) are evaluated by applying isodesmic reactions at both BMC-CCSD//BB1K/6-31+G(d, p) and MC-QCISD//BB1K/6-31+G(d, p) levels of theory.




Similar content being viewed by others
References
Garland NL, Medhurst LJ, Nelson HH (1993) J Geophys Res 98:23107. doi:10.1029/93JD02550
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, Jenkin ME, Rossi MJ, Troe J, Wallington TJ (2008) Atmos Chem Phys 8:4141
Sander SP, Ravishankara AR, Golden DM, Kolb CE, Kurylo MJ, Molina MJ, Moortgat GK, Finlayson-Pitts BJ, Wine PH, Huie RE (2006) JPL Publ 06:2
Nelson DD Jr, Zahniser MS, Kolb CE, Magid H (1995) J Phys Chem 99:16301. doi:10.1021/j100044a016
Hsu K-J, DeMore WB (1995) J Phys Chem 99:1235. doi:10.1021/j100004a025
Zhang Z, Padmaja S, Saini RD, Huie RE, Kurylo MJ (1994) J Phys Chem 98:4312. doi:10.1021/j100067a017
Mogelberg TE, Feilberg A, Biessing AMB, Sehested J, Bilde M, Wallington TJ, Nielsen OJ (1995) J Phys Chem 99:17386. doi:10.1021/j100048a013
Truhlar DG (1995) In: Heidrich D (ed) The reaction path in chemistry: current approaches and perspectives. Kluwer, Dordrecht, pp 229
Truhlar DG, Garrett BC, Klippenstein SJ (1996) J Phys Chem 100:12771. doi:10.1021/jp953748q
Hu W-P, Truhlar DG (1996) J Am Chem Soc 118:860. doi:10.1021/ja952464g
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian Inc., Pittsburgh
Zhao Y, Lynch BJ, Truhlar DG (2004) J Phys Chem A 108:2715. doi:10.1021/jp049908s
Becke AD (1988) Phys Rev A 38:3098. doi:10.1103/PhysRevA.38.3098
Becke AD (1996) J Chem Phys 104:1040. doi:10.1063/1.470829
Lynch BJ, Zhao Y, Truhlar DG (2005) J Phys Chem A 109:1643. doi:10.1021/jp045847m
Chuang YY, Corchado JC, Truhlar DG (1999) J Phys Chem A 103:1140. doi:10.1021/jp9842493
Corchado JC, Chuang YY, Fast PL, Hu WP, Liu YP, Lynch GC, Nguyen KA, Jackels CF, Ramos AF, Ellingson BA, Lynch BJ, Melissas VS, Villa J, Rossi I, Coitino EL, Pu J, Albu TV (2005) POLYRATE, version 9.3.1. University of Minnesota, Minneapolis
Garrett BC, Truhlar DG (1979) J Chem Phys 70:1593. doi:10.1063/1.437698
Garrett BC, Truhlar DG (1979) J Am Chem Soc 101:4534. doi:10.1021/ja00510a019
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730 Erratum: (1983) 87: 4554
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440. doi:10.1021/ar50156a002
Truhlar DG, Isaacson AD, Garrett BC (1985) Generalized transition state theory. In: Baer M (ed) The theory of chemical reaction dynamics, vol 4. CRC Press, Boca Raton, p 65
Truhlar DG, Garrett BC (1984) Annu Rev Phys Chem 35:159. doi:10.1146/annurev.pc.35.100184.001111
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu YP, Grarrett BC, Steckler R, Issacson AD, Rai SN, Hancock GC, Lauderdale JG, Joseph T, Truhlar DG (1992) Comput Phys Commun 71:235. doi:10.1016/0010-4655(92)90012-N
Liu Y-P, Lynch GC, Truong TN, Lu D-H, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408. doi:10.1021/ja00059a041
Garrett BC, Truhlar DG, Wagner AF, Dunning TH (1983) J Chem Phys 78:4400. doi:10.1063/1.445323
Garrett BC, Abusalbi N, Kouri DJ, Truhlar DG (1985) J Chem Phys 83:2252. doi:10.1063/1.449318
Taghikhani M, Parsafar GA, Sabzyan H (2005) J Phys Chem A 109:8158. doi:10.1021/jp0524173
Galano A, Alvarez-ldaboy JR, Ruiz-Santoyo ME, Vivier-Bunge A (2004) Chem Phys Chem 5:1379. doi:10.1002/cphc.200400127
Gao H, Wang Y, Liu J, Yang L, Li Z, Sun C (2008) Phys Chem A 112:4176. doi:10.1021/jp077611z
Truhlar DG (1991) J Comput Chem 12:266. doi:10.1002/jcc.540120217
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221. doi:10.1063/1.480768
Fast PL, Truhlar DG (2000) J Phys Chem A 104:6111. doi:10.1021/jp000408i
Lide DR (1999) CRC Handbook of Chemistry and Physics, 80th edn. CRC Press, New York
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure, IV: constants of diatomic molecules. Van Nostrand Reinhold, New York
Shimanouchi T (1972) Tables of molecular vibrational frequencies consolidated, vol 1. National Bureau of Standards U.S. GPO, Washinton
Chase MW, Davies CA, Downey JR, Frurip DJ, MacDonald RA, Syverud AN (1985) JANAF, vol 14 (Suppl 1); American Chemical Society, Washington DC
Albu TV, Swaminathan S (2006) J Phys Chem A 110:7663. doi:10.1021/jp0615454
Chemistry Webbook NIST, Linstrom PJ, Mallard WG (eds) Available from: http://webbook.Nist.Gov/chemistry
Miller WH (ed) (1976) Dynamics of molecular collisions, Part B, vol. 2 of Modern theoretical chemistry, Plenum Press, New York
Gilbert RG, Smith SC (1990) Theory of unimolecular and recombination reactions. Blackwell, Oxford
Allison TC, Truhlar DG (1998) In: Thompson DL (ed) Modern methods for multidimensional dynamics computations in chemistry. World Scientific, Singapore. p 618
Taghikhani M, Parsafar GA (2007) J Phys Chem A 111:8095. doi:10.1021/jp072403s
Acknowledgments
We thank Professor Donald G. Truhlar for providing the POLYRATE 9.3 program. This work was supported by the National Natural Science Foundation of China (20303007, 20333050, and 20073014), the Program for New Century Excellent Talents in University (NCET). The authors are grateful to the referees for their valuable comments on improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, H., Wang, Y., Wang, Q. et al. Hydrogen abstraction from CF3CF2CFH2 and CF3CFHCF2H by OH radicals and Cl atoms: theoretical enthalpies and rate constants. Theor Chem Acc 124, 59–70 (2009). https://doi.org/10.1007/s00214-009-0581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0581-5