Abstract
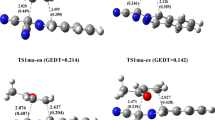
The phospha-Wittig reaction HP=PH3 + O=CHX → HP=CHX + O=PH3 (X = H, F, Cl, Me, OMe, NMe2, CMe3) was examined using the density functional theory calculations. All of the structures were completely optimized at the B3LYP/6-311++G** level of theory. The reactivities of various O=CHX were examined by estimating their activation energies. The main finding of this work is that the configuration mixing model can successfully predict the relative ordering of the activation energy and reaction enthalpies of the phospha-Wittig reaction. It was demonstrated that O=CHX with more electro-releasing substituents will possess a smaller singlet–triplet splitting. This will facilitate the phospha-Wittig reaction and will result in a larger exothermicity.







Similar content being viewed by others
References
Maryanoff BE, Reitz AB (1989) Chem Rev 89:863–927. doi:10.1021/cr00094a007
Volatron F, Eisenstein O (1987) J Am Chem Soc 109:2–14. doi:10.1021/ja00235a001
Hoffmann RW (2001) Angew Chem Int Ed 40:1411–1416. doi:10.1002/1521-3773(20010417)40:8<1411::AID-ANIE1411>3.0.CO;2-U
Liao HY, Yen MY (2008) N J Chem 32:511–516. doi:10.1039/b715049k
Keith B, Dillon FM, Nixon JF (1998) Phosphorus: the carbon copy: from organophosphorus to phospha-organic chemistry. Wiley, Chichester
Shah S, Protasiewicz JD (2000) Coord Chem Rev 210:181–201. doi:10.1016/S0010-8545(00)00311-8
Marinetti A, Mathey F (1988) Angew Chem Int Ed Engl 27:1382–1384. doi:10.1002/anie.198813821
Marinetti A, Bauer S, Ricard L, Mathey F (1990) Organometallics 9:793–798. doi:10.1021/om00117a040
Marinetti A, Ricard L, Mathey F (1990) Organometallics 9:788–793. doi:10.1021/om00117a039
Shah S, Yap GPA, Protasiewicz JD (2000) J Organomet Chem 608:12–20. doi:10.1016/S0022-328X(00)00284-9
Shah S, Protasiewicz JD (1998) Chem Commun (Camb) 1585–1586. doi:10.1039/a802722f
Mracec M, Pascariu A, Berger S, Mracec M (2007) Int J Quantum Chem 107:1782–1793. doi:10.1002/qua.21298
Molander GA, Ham J, Canturk B (2007) Org Lett 9:821–824. doi:10.1021/ol063043e
El-Batta A, Jiang CC, Zhao W, Anness R, Cooksy AL, Bergdahl M (2007) J Org Chem 72:5244–5259. doi:10.1021/jo070665k
Robiette R, Richardson J, Aggarwal VK, Harvey JN (2006) J Am Chem Soc 128:2394–2409. doi:10.1021/ja056650q
Yu XC, Huang X (2002) Synlett 1895–1897
Lu WC, Wong NB, Zhang RQ (2002) Theor Chem Acc 107:206–210. doi:10.1007/s00214-001-0320-z
Balema VP, Wiench JW, Pruski M, Pecharsky VK (2002) J Am Chem Soc 124:6244–6245. doi:10.1021/ja017908p
Wang Q, El Khoury M, Schlosser M (2000) Chem Eur J 6:420–426. doi:10.1002/(SICI)1521-3765(20000204)6:3<420::AID-CHEM420>3.0.CO;2-H
Yamataka H, Nagase S (1998) J Am Chem Soc 120:7530–7536. doi:10.1021/ja974237f
Yamataka H, Nagareda K, Takatsuka T, Ando K, Hanafusa T, Nagase S (1993) J Am Chem Soc 115:8570–8576. doi:10.1021/ja00072a008
Seth M, Senn HM, Ziegler T (2005) J Phys Chem A 109:5136–5143. doi:10.1021/jp045318i
Smith RC, Chen XF, Protasiewicz JD (2003) Inorg Chem 42:5468–5470. doi:10.1021/ic0345471
Mathey F (2003) Angew Chem Int Ed 42:1578–1604. doi:10.1002/anie.200200557
Shah S, Simpson MC, Smith RC, Protasiewicz JD (2001) J Am Chem Soc 123:6925–6926. doi:10.1021/ja015767l
Power PP (1993) Angew Chem Int Ed Engl 32:850–851. doi:10.1002/anie.199308501
Cummins CC, Schrock RR, Davis WM (1993) Angew Chem Int Ed Engl 32:756–759. doi:10.1002/anie.199307561
Marinetti A, Ricard L, Mathey F (1992) Synthesis (Stuttgart) 1–2:157–162
Marinetti A, Lefloch P, Mathey F (1991) Organometallics 10:1190–1195. doi:10.1021/om00050a061
Becke AD (1993) J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Becke AD (1992) J Chem Phys 97:9173–9177. doi:10.1063/1.463343
Becke AD (1992) J Chem Phys 96:2155–2160. doi:10.1063/1.462066
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654. doi:10.1063/1.438955
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269. doi:10.1063/1.447079
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03. Gaussian, Inc, Pittsburgh
Baerends EJ, Gritsenko OV (1997) J Phys Chem A 101:5383–5403. doi:10.1021/jp9703768
Laird BB, Ross RB, Ziegler T (eds) (1996) Chemical applications of density-functional theory. American Chemical Society, Washington
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1996) NBO 4.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Foresman JB, Keith TA, Wiberg KB, Snoonian J, Frisch MJ (1996) J Phys Chem 100:16098–16104. doi:10.1021/jp960488j
Hammond GS (1955) J Am Chem Soc 77:334–338. doi:10.1021/ja01607a027
Shaik SS, Schlegel HB, Wolfe S (1992) Theoretical aspects of physical organic chemistry: the Sn2 mechanism. Wiley, New York
Su MD, Liao HY, Chung WS, Chu SY (1999) J Org Chem 64:6710–6716. doi:10.1021/jo990504j
Acknowledgments
The author is grateful to the National Center for High-performance Computing for computer time and facilities. The author also thank the National Science Council of Taiwan for financial support (NSC 97-2113-M-152-001-MY2) and Prof. Dr. San-Yan Chu for helpful and generous suggestions. Also, the author acknowledges the referees for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, HY. Computational study of the substitution effect on the mechanism for phospha-Wittig reaction. Theor Chem Acc 124, 49–57 (2009). https://doi.org/10.1007/s00214-009-0579-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0579-z