Abstract
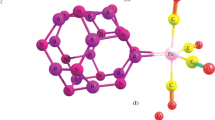
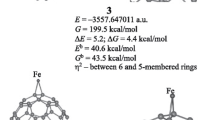
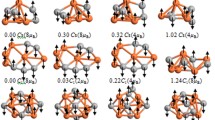
The search for stable structures of neutral Fe2C3 particle was based on the geometry optimization of the known FeC3 and Fe2C2 isomers with the Fe and C atoms approaching from various directions. The geometry optimization of more than 2,000 initial structures was carried out using the DFT based DMol3 method and converged to 41 stable configurations. The structures containing C3 triangle and the cyclic planar isomer with transannular bonds are found to have the lowest binding energies. The effective charges and total spin densities on the atoms were calculated using integral scheme incorporated in DVM and Hirshfeld procedure of DMol3. The relations between geometrical structures and spin moments ordering are discussed. For the evaluation of potential barriers the geometry optimization of all Fe2C3 configurations was performed with a thermal occupation, corresponding to the various values of the excitation energy.
Similar content being viewed by others
References
Guo BC, Kerns KP and Castleman AW (1992). Science 255: 1411–1413
Rohmer MM, Benard M and Poblet JM (2000). Chem Rev 100: 495–542
Liu P and Rodriguez JA (2004). J Chem Phys 120: 5414–5423
Noya EG, Longo RC and Gallego LJ (2003). J Chem Phys 119: 11130–11134
Harris H and Dance I (2007). Polyhedron 26: 250–265
Pilgrim JS and Duncan MA (1993). J Am Chem Soc 115: 6958–6961
Kan SZ, Lee SA and Freiser BS (1996). J Mass Spectrom 31: 62–68
Von Heldem G, Tielens AGGM, Van Heijnsbergen D, Duncan MA, Hony S, Waters LBFM and Meijer G (2000). Science 288: 313–316
Ryzhkov MV, Ivanovskii AL and Delley BT (2005). Chem Phys Lett 404: 400–408
Goedecker S, Hellmann W and Lenosky T (2005). Phys Rev Lett 95: 055501
Hellmann W, Hennig RG, Goedecker S, Umrigar CJ, Delley B and Lenosky T (2007). Phys Rev B 75: 08541
Dmol3 β version (1997) Molecular simulations, San Diego
Perdew JP, Burke S and Ernzerhof M (1996). Phys Rev Lett 77: 3865–3868
Mulliken RS (1955). J Chem Phys 23: 1833–1840
Hirshfeld FL (1977). Theor Chim Acta 44: 129–138
Baerends EJ, Ellis DE and Ros P (1973). Chem Phys 2: 41–51
Press MR and Ellis DE (1987). Phys Rev B 35: 4438–4454
Ryzhkov MV (1998). J Struct Chem 39: 933–937
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ and Fiolhais C (1992). Phys Rev B 46: 6671–6687
Perdew JP (1991). Phys B 172: 1–6
Lee C, Yang W and Parr RG (1988). Phys Rev B 37: 785–789
Becke AD (1988). Phys Rev A 38: 3098–3100
Delley B (2006). J Phys Chem A 110: 13632–13639
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryzhkov, M.V., Ivanovskii, A.L. & Delley, B. Geometry, electronic structure and energy barriers of all possible isomers of Fe2C3 nanoparticle. Theor Chem Account 119, 313–318 (2008). https://doi.org/10.1007/s00214-007-0385-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-007-0385-4