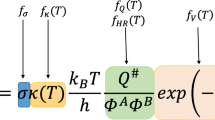Abstract
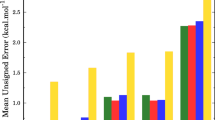
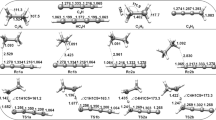
The kinetics of the hydrogen abstraction at alkanes by formyl radicals is investigated using the reaction class transition state theory (RC-TST) approach combined with the linear energy relationship (LER) or the barrier height grouping (BHG). The rate constants of a reaction in this class can be estimated through those of the reference reaction, CHO + C2H6, which are obtained from rate constants of the reaction that involves the smallest species, namely CHO + CH4, using the explicit RC-TST scaling. The thermal rate constants of this smallest reaction are evaluated at the canonical variational transition state theory (CVT) with the corrections from the small-curvature tunneling (SCT) and hindered rotation (HR) treatments. Our analyses indicate that less than 40% systematic errors, on the average, exist in the predicted rate constants using both the LER approach, where only reaction energy is needed, and the BHG approach, where no additional information is needed; while comparing to explicit rate calculations the differences are less than 60%.
Similar content being viewed by others
References
Curran HJ, Gaffuri P, Pitz WJ and Westbrook CK (2002). Combust Flame 129: 253
Zhang H-Y and McKinnon JT (1995). Combust Sci Tech 107: 261
Richter H and Howard JB (2002). Phys Chem Chem Phys 4: 2038
Richter H, Granata S, Green WH and Howard JB (2005). Proc Combust Inst 30: 1397
Tsang W (1988). J Phys Chem Ref Data 17: 887
Tsang W and Hampson RF (1986). J Phys Chem Ref Data 15: 1087
Tsang W (1990). J Phys Chem Ref Data 19: 1
Zhang S and Truong TN (2003). J Phys Chem A 107: 1138
Truong TN (2000). J Chem Phys 113: 4957
Huynh LK, Ratkiewicz A and Truong TN (2006). J Phys Chem A 110: 473
Kungwan N and Truong TN (2005). J Phys Chem A 109: 7742
Truong TN, Duncan WT and Tirtowidjojo M (1999). Phys Chem Chem Phys 1: 1061
Truong TN and Truong TTT (1999). Chem Phys Lett 314: 529
Truong TN, Maity DK and Truong T-TT (2000). J Chem Phys 112: 24
Polanyi JC (1972). Acc Chem Res 5: 161
Evans MG and Polanyi M (1936). Trans Faraday Soc 32: 1333
Evans MG and Polanyi M (1936). Proc R Soc 154: 133
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr. TV, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision A.1, Gaussian, Inc., Pittsburgh
Becke AD (1993). J Chem Phys 98: 1372
Lee C, Yang W and Parr RG (1988). Phys Rev 37: 785
Truong TN (1994). J Chem Phys 100: 14
Truong TN and Duncan W (1994). J Chem Phys 101: 7408
Lynch BJ, Fast PL, Harris M and Truhlar DG (2000). J Phys Chem A 104: 4811
Dunning TH (1989). J Chem Phys 94: 5523
Gonzalez C and Schlegel HB (1989). J Chem Phys 90: 2154
Gonzalez C and Schlegel HB (1990). J Phys Chem 94: 5523
Truhlar DG and Garrett BC (1980). Accs Chem Res 13: 440
Truhlar DG, Isaacson AD and Garrett BC (1985). Generalized Transition State Theory. In: Baer, M (eds) Theory of chemical reaction dynamics, vol 4, pp 65. CRC Press, Boca Raton
Truhlar DG (1995). Direct dynamics method for the calculation of reaction rates. In: Heidrich, D (eds) The reaction path in chemistry: current approaches and perspectives, pp 229. Kluwer Academic, Dordrecht
Duncan WT, Bell RL and Truong TN (1998). J Comp Chem 19: 1039
Pople JA, Head-Gordon M and Raghavachari K (1987). J Chem Phys 87: 5968
Truong TN http://www.cseo.net
Ayala PY and Schlegel HB (1998). J Chem Phys 108: 2314
Ochterski JW, Petersson GA, Montgomery JA Jr (1996). J Chem Phys 104: 2598
NIST Standard Reference Database Number 69, June 2005 Release, http://webbook.nist.gov/chemistry/
Miller WH (1979). J Am Chem Soc 101: 6810
DeMore WB, Sander SP, Golden DM, Hampson RF, Kurylo MJ, Howard CJ, Ravishankara AR, Kolb CE, Molina MJ (1992) in JPL publication (Jet Propulsion Laboratory, Pasadena), pp 90
Author information
Authors and Affiliations
Corresponding author
Additional information
Contribution to Mark S. Gordon 65th Birthday Festschrift Issue.
Rights and permissions
About this article
Cite this article
Huynh, L.K., Truong, T.N. Kinetics of the hydrogen abstraction CHO + Alkane → HCHO + Alkyl reaction class: an application of the reaction class transition state theory. Theor Chem Account 120, 107–118 (2008). https://doi.org/10.1007/s00214-007-0311-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-007-0311-9