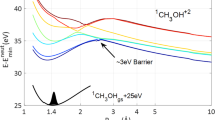
Abstract
This article shows that by using ab initio or first principle calculations it is possible to obtain reliable ingredients needed to simulate pump-probe and optimal control experiments. Our experimental challenge is to elucidate the reaction mechanism behind an optimal pulse tailored to maximize ionization in the system CpMn(CO)3, while avoiding CO dissociation. Starting from MRCI/CASSCF potential energy curves calculated along the relevant CO fragmentation channel, we use the resulting MRCI wave function to estimate non-adiabatic couplings, as well as neutral-to-neutral and neutral-to-ionic dipole couplings. The state-of-the-art potentials and couplings serve to perform wave packet propagations which simulate the femtosecond pump-probe spectra that explain the features shown in the experimental optimal pulse.
Similar content being viewed by others
References
Zewail AH (2000). (Nobel lecture) Angew Chem Int Ed 39:2586
Crim F (1990). Science 249:1387
Shapiro M, Brumer P (1986). Chem Phys Lett 126:541
Tannor DJ, Rice SA (1985). J Chem Phys 83:5013
Tannor DJ, Kosloff R, Rice SA (1986). J Chem Phys 85:5805
Bergmann K, Theuer H, Shore BW (1998). Rev Mod Phys 70:1003
Rabitz H, de Vivie-Riedle R, Motzkus M, Kompa K (2000). Science 288:824
Baumert T, Thalweiser R, Weiss V, Gerber G (1995). Femtosecond chemistry. VCH, Weinheim
Zhu L, Suto K, Fiss J, Wada R, Seidan T, Gordon RJ (1997). Phys Rev Lett s78:4108
Thompson DL (1998). Modern methods for multidimensional dynamics computations in chemistry. World Scientific, Singapore
Kühn O, Manz J, Miller WH (eds). (2004). Multidimensional quantum reaction dynamics, vol 304 (1–2), Chem Phys (special issue).
Judson RS, Rabitz H (1992). Phys Rev Lett 68:1500
Brixner T, Gerber G (2003). Chem Phys Chem 4:418
Bardeen CJ, Yakovlev V, Wilson K, Carpenter S, Weber PM, Warren W (1997). Chem Phys Lett 280:151
Assion A, Baumert T, Bergt M, Brixner T, Kiefer B, Seyfried V, Strehle M, Gerber G (1998). Science 282:919
Brixner T, Damrauer NH, Gerber G, Niklaus P (2001). Nature 414:57
Glaß A, Rozgonyi T, Feurer T, Szabó G, Sauerbrey R (2000). Appl Phys B71:267
Levis RJ, Menkir GM, Rabitz H (2001). Science 292:709
Vajda Š et al (2002). Ultrafast dynamics in molecular science. World Scientific, Singapore
Herek JL, Wohlleben W, Cogdell RJ, Zeidler D, Motzkus M (2002). Nature 417:533
Geremia JM, Zhu WS, Rabitz H (2000). J Chem Phys 113:10841
Hornung T, Motzkus M, de Vivie-Riedle R (2001). J Chem Phys 115:3105–3110
Hornung T, Motzkus M, de Vivie-Riedle R (2002). Phys Rev A 65:021403
Kurtz L, Rabitz H, de Vivie-Riedle R (2002). Phys Rev A 65:032514
Zhu W, Rabitz H (1999). J Chem Phys 111:472
Mitra A, Rabitz H (2003). Phys Rev A 67:033407
White JL, Pearson BJ, Bucksbaum PH (2004). quant-ph/0401018
Mancal T, May V (2002). Chem Phys Lett 362:407
Daniel C, Full J, González L, Lupulescu C, Manz J, Merli A, Vajda S, Wöste L (2003). Science 299:536
Manz J, Wöste L (eds). (1995). Femtosecond chemistry. VCH, Weinheim
Eickeyer F, Kaindl RA, Woerner M, Elsaesser T, Weiner AM (2000). Opt Lett 25:1472
Witte T, Hornung T, Windhourn L, Proch D, de~Vivie-Riedle R, Motzkus M, Kompa KL (2003). J Chem Phys 118:2021
Olivucci M (eds). (2005). Computational photochemistry. Elsevier, Amsterdam
Banares L, Baumert T, Bergt M, Kiefer B, Gerber G (1997). Chem Phys Lett 267:141
Trushin SA, Fuss W, Schmid WE, Kompa L (1998). J Phys Chem 102:4129
Trushin SA, Fuss W, Kompa L, Schmid W (2000). Chem Phys 259:313
Matsubara T, Daniel C, Veillard A (1994). Organometallics 13:4905
Daniel C, Kolba E, Lehr L, Manz J, Schröder T (1994). J Phys Chem 98:9823
Finger K, Daniel C, Saalfrank P, Schmidt B (1996). J Phys Chem 100:3368
Erdman M, Rubner O, Shen Z, Engel V (2001). Chem Phys Lett 341:338
Rubner O, Engel V (2001). J Chem Phys 115:2936
Paterson MJ, Hunt PA, Robb MA, Takahashi O (2002). J Phys Chem 106:10494
Trushin SA, Fuss W, Schmid W (2004). J Chem B 37:3987
Full J, Daniel C, González L (2001). J Phys Chem A 105:184
Full J, Daniel C, González L (2003). Phys Chem Chem Phys 5:87
Daniel C, Full J, González L, Kaposta C, Krenz M, Lupulescu C, Manz J, Minoto S, Oppel M, Rosendo-Francisco P, Vajda Š, Wöste L (2001). Chem Phys 267:247
Hirsch G, Bruna PJ, Buenker RJ, Peyerimhoff SD (1980). Chem Phys 45:335
Baer M (1975). Chem Phys Lett 35:112
Full J, González L, Manz J (2005). Chem Phys 314:143
Schön J, Köppel H (1999). J Phys Chem 103:8579
Rabitz H (2003). Science 299:525
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González, L., Full, J. A first principles approach to optimal control. Theor Chem Acc 116, 148–159 (2006). https://doi.org/10.1007/s00214-005-0035-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-005-0035-7